Article contents
Modification with ultrasonication for enhanced properties of cobalt-based zeolitic imidazolate framework
Published online by Cambridge University Press: 28 August 2018
Abstract
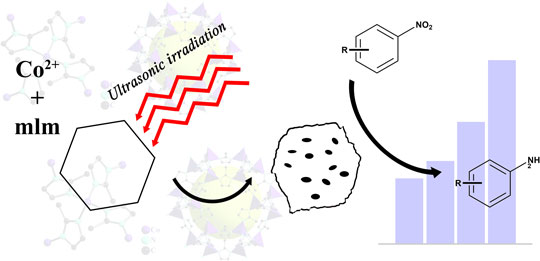
Effective modification of existing supported catalyst has attracted plenty of interests recently. Herein, we introduced ultrasonication to synthesize the palladium-loaded cobalt-based zeolitic imidazolate framework and compared its properties with those using the conventional method. Remarkably, the ultrasonicated frameworks possess 15.33% higher of Brunauer–Emmett–Teller (BET) surface area and 63.37% higher of t-plot external surface area, respectively, which lead up to 23% rise in the degradation of organic pollutants under optimized conditions. Characterizations clearly revealed the causality between ultrasonication, morphology, and catalytic performance compared with their non-ultrasonicated counterparts which further demonstrates a simple but useful method for the modification of supported catalysts.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 3
- Cited by



