Article contents
Improving ambient stability of BiI3-based perovskites using different phosphoniums as the organic cation
Published online by Cambridge University Press: 30 April 2018
Abstract
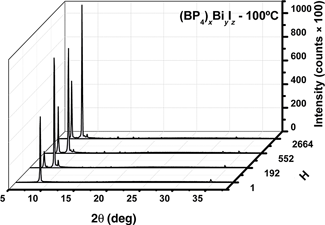
Perovskites solar cells have reached impressive efficiencies (22%) in recent years. Because certain environmental concerns are raised by the use of lead halides, there is an interest to seek out lead-free alternatives, featuring bismuth or antimony. Alongside, one of the major drawbacks displayed by MAPbI3 is their low stability at ambient conditions. In this work, (RP4)xBiyIz were synthesized, using different types of tetra-alkylphosphoniums (R4PI) were R = ethyl, butyl, hexyl, and octyl, to assess their stability. Afterwards, they were characterized to study their morphology and crystal structure, as well as their optical properties.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 5
- Cited by



