Published online by Cambridge University Press: 14 August 2017
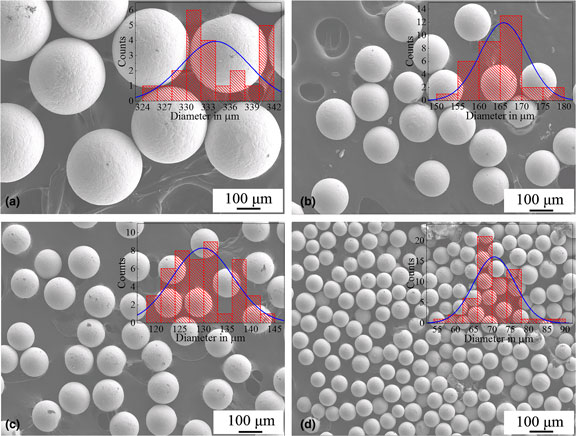
Spherical Sn0.3Ag0.7Cu (wt.%) solder droplets with diameter ranging from 70.6 to 334.0 µm were prepared using pulsated orifice ejection method. Compared with conventional atomization, these droplets are almost completely spherical with a much narrower size distribution. The surface of these droplets is smooth without detectable satellite particles. Furthermore, both the composition and microstructure are homogenous throughout any single droplet regardless of their size. Detailed microstructural analysis shows that nano-sized Ag3Sn particles are distributed homogenously in the β-Sn matrix. The results suggest that the droplets have advantage as electronic packaging material and be a promising candidate material for three-dimensional printing.