Article contents
Engineering CAR-expressing natural killer cells with cytokine signaling and synthetic switch for an off-the-shelf cell-based cancer immunotherapy
Published online by Cambridge University Press: 27 March 2019
Abstract
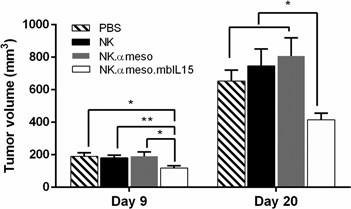
Immune cells can be genetically engineered with a synthetic chimeric antigen receptor (CAR) to eliminate cancer cells, but clinical efficacy in solid tumors has been disappointing due in part to the immunosuppressive tumor microenvironment (TME). Additionally, the cost and logistical issues of personalized medicine necessitate the creation of an off-the-shelf CAR therapy. Synthetic biology tools were implemented in addressing these problems: an anti-mesothelin CAR, membrane-bound IL-15/IL-15Rα complex, and inducible caspase 9 “kill switch” were expressed in natural killer cells for tumor-targeting capabilities, immunostimulatory effects, and safety in treating a preclinical model of ovarian cancer with a renewable, allogenic cell therapy.
- Type
- Synthetic Biology Research Letter
- Information
- Copyright
- Copyright © Materials Research Society 2019
Footnotes
Authors contributed equally to this work.
References
- 2
- Cited by


