Published online by Cambridge University Press: 25 July 2011
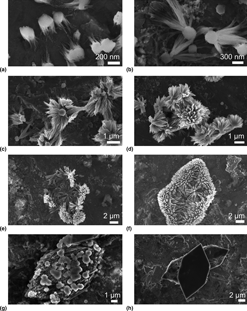
Molecules self-assembling in solution may pass through multiple phases and morphologies before reaching a thermodynamically stable state. Here we demonstrated this effect in tetracyanoquinodimethane (TCNQ), an organic molecule often used as an electron acceptor in charge transfer complex compounds. We showed that it self-assembles in a wide range of crystal habits, from nanocoils to polyhedral crystals. Scanning electron microscopy imaging on freeze-dried samples revealed the crystal growth of TCNQ starting from seed-shaped nucleation sites, progressing through flower-like structures and finally forming polyhedral micro-crystals. These results are supplemented by absorption spectroscopy as well as x-ray powder diffraction (XRPD) characterization of a powder sample.