Published online by Cambridge University Press: 13 December 2013
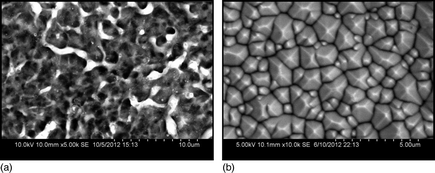
Plasma electrolysis (PE) is a combination of electrolysis and plasma discharge. Previous studies indicated that PE usually created porous surface with irregular morphology as a result of the plasma–cathode interaction that was dominated by physical reactions. This paper demonstrated that highly ordered textured silicon surfaces could be created using PE. This abnormal anisotropic etching phenomenon implied that the chemical reactions were decoupled from the physical processes and the physical reactions were suppressed. Raman spectra confirmed that the textured silicon surface created by PE conserved the crystalline structure. Therefore, PE may lead to new process regimes for surface engineering.