Article contents
Commercial carbon anode material surface-modified by spinel lithium titanate for fast lithium-ion interaction
Published online by Cambridge University Press: 16 December 2019
Abstract
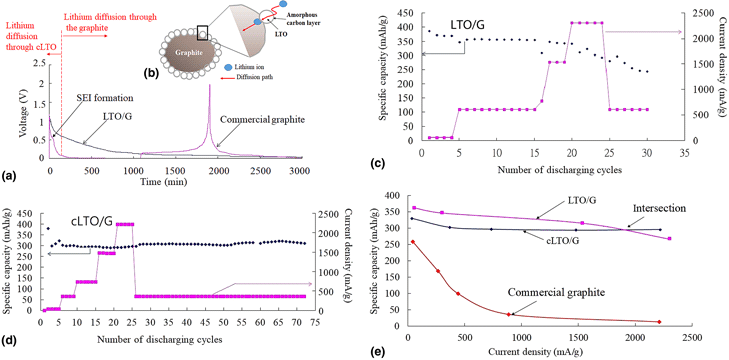
This research utilizes anatase TiO2 incorporated with lithium salt via a simple wet physical method to surface-modified the commercial graphite to form the lithium titanate/graphite composite coated with an amorphous carbon layer on its surface (the double core–shell structure) to enhance its surface conductivity. This double core–shell structure provides a stable specific capacity about 280 mAh/g under the high current density, 2.25 A/g with 15% capacity retention decay. Its intercalation potential is below 1 V that is much lower than that of 1.55 V, the intercalation potential of spinel Li4Ti5O12, to make higher power and energy density for a full cell.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 2
- Cited by



