No CrossRef data available.
Published online by Cambridge University Press: 19 March 2018
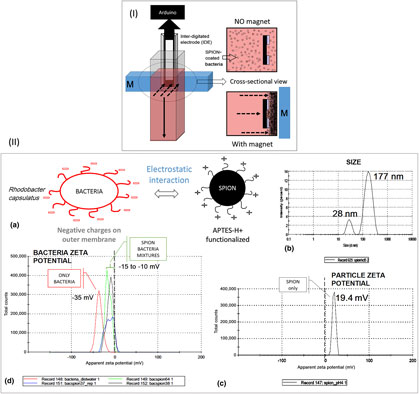
Magnetic field has been used to trigger biofilm formation. Iron oxide nanoparticles were attached to bacterial cells and cells were aggregated by application of magnetic field. Artificial cellular crowding triggered quorum sensing and led to the formation of biofilm at the sub-threshold population. Aggregation process was monitored by studying temporal dynamics of capacitance and conductance profiles. Capacitive profile exhibited a plateau upon introduction of magnetic field which was retained even after field was removed. This hysteresis property signified biofilm initiation in response to artificial crowding. This work demonstrates how synthetic biology is enabled by including nanoparticles in the interactome.