Article contents
Single precursor synthesis of copper sulfide nanocrystals using aerosol spray pyrolysis
Published online by Cambridge University Press: 19 March 2013
Abstract
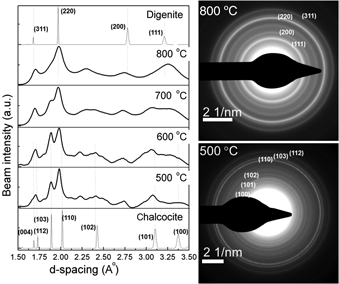
We have investigated the feasibility of aerosol spray pyrolysis for the synthesis of copper sulfide nanocrystals, which are promising candidates for the development of low-cost, printable photovoltaic devices. A solution of copper diethyldithiocarbamate in toluene is aerosolized and aerodynamically dragged through a tube furnace, where the droplets are dried and nanocrystals are formed. Particles smaller than 20 nm are produced. The particles are preferentially formed as digenite (Cu1.8S), although we show that with low furnace temperature it is possible to produce chalcocite (Cu2S) nanocrystals.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
- 1
- Cited by


