Article contents
The need for advanced three-dimensional neural models and developing enabling technologies
Published online by Cambridge University Press: 10 July 2017
Abstract
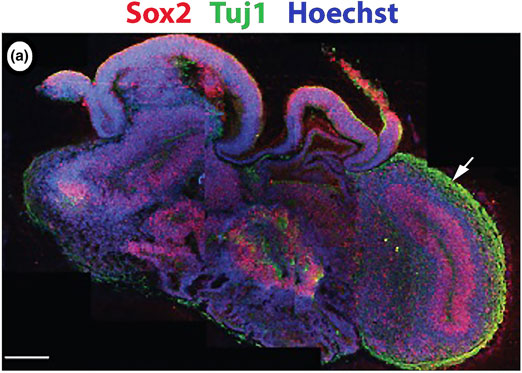
Neurological and psychiatric disorders account for an increasing proportion of the global disease burden. Correspondingly the neuropharmaceutical industry has experienced a significant contraction in recent years resulting in a poor variety of therapies available to treat an expanding range of conditions. Perhaps the greatest contributor to this failure in drug-discovery is the lack of understanding of the underlying biology of the nervous system and how molecular scale events translate into macroscale pathologies. Due to the unique nature of the human nervous system commonly used model organisms are often poorly representative of human pathologies resulting in a need for the development of advanced in vitro models that are capable of faithfully modeling complex structures within the brain. In this prospective, strategies for the generation of neuronal circuits and cultivation of complex three-dimensional (3D) cultures are explored. Frequently these constructs provide valuable insights into systems and processes that are difficult to explore in vivo due to the isolated and delicate nature of neuronal tissues. New developments are required to assess the physiological functions of 3D tissues in vitro.
- Type
- Biomaterials for 3D Cell Biology Prospective Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 7
- Cited by


