Published online by Cambridge University Press: 22 April 2020
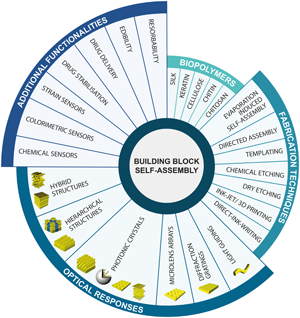
Natural systems displaying optical properties have for long been an inspiration for new classes of optical constructs. Using the same families of materials employed by Nature in combination with their directed assembly allows access to n-dimensions of control to, ultimately, generate optical systems with multiple coexisting functions. This review provides an overview of lab-made optical systems made of protein and polysaccharide-derived materials found in naturally occurring optical systems. Recent advances in optical biomimicry and bioinspired, polyfunctional optical structures are presented, addressing attributes such as sensing, edible devices, biologically activity, and resorbable optical formats.