Article contents
Alkyne-modified water-stable alkylammonium lead(II) iodide perovskite
Published online by Cambridge University Press: 16 April 2018
Abstract
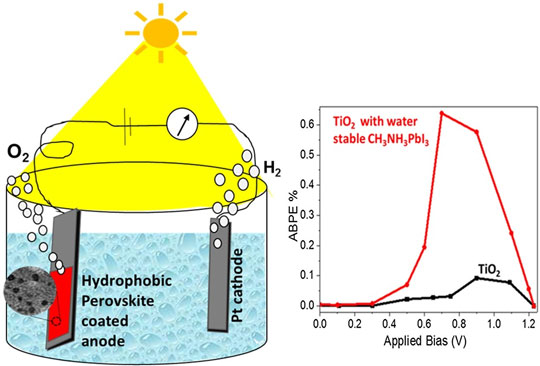
Perovskite materials are sensitive to environmental conditions. Here we report the synthesis and characterization of a hydrophobic alkylammonium lead(II) iodide perovskite with enhanced stability in water. Water stability was achieved by growing a shell of 4-[(N-3-butyne)carboxyamido]anilinium lead(II) iodide over methylammonium lead(II) iodide. As a proof of concept, the water-splitting reaction was performed using our new material coated on TiO2, and a 7-fold increase in applied bias photon-to-current efficiency was observed as compared with standard p25-TiO2. Such simple and versatile chemical modification to induce high water stability is useful toward exploring new applications for the perovskite materials.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 2
- Cited by



