Published online by Cambridge University Press: 12 June 2018
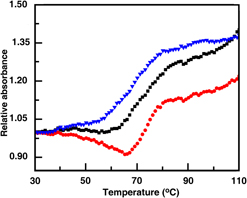
In this study, nano-hydroxyapatite (n-HAp) of average crystallite size ∼8.15 ± 4 nm of hexagonal geometry with size ranging between 14 and 50 nm was synthesized in laboratory at room temperature by using suitable sources of calcium and phosphate ions and using triethanolamine. Mesoporous bioactive glass (MBG) was synthesized by using cationic surfactant cetyl trimethyl ammonium bromide of the SiO2–CaO–P2O5 glass system. After calcination at 650 °C, MBG powders were having a zeta potential of −16.5 mV (pH ∼9.1), median particle size ∼75 nm, and specific surface area 473.2 m2/g. An aqueous suspension of DNA was used to disperse both n-HAp and MBG and further subjected for analysis including absorbance, circular dichroism spectroscopy, UV-melting, and isothermal titration calorimetry. Absorbance spectroscopy indicated that an equilibrium binding was obtained between both materials and DNA in solution phase. Due to the addition of the nanomaterial, molar ellipticity of DNA was changed revealing that the materials were interacted with DNA. From UV melting characterization, there is a shifting of the melting temperature of DNA in the presence of MBG and n-HAp, respectively, suggesting that the nanoparticles stabilized DNA helix to a considerable extent.