Article contents
A novel, green, and biocompatible graphene-based carbonaceous material for immobilization of cytochrome c
Published online by Cambridge University Press: 09 November 2018
Abstract
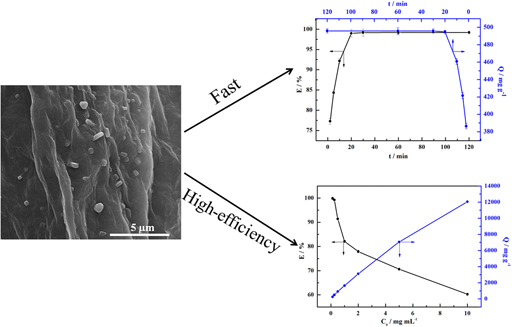
The immobilization of cytochrome c (cyt c) on tea polyphenol functionalized and reduced graphene oxide (TPG) was carried out by a simple adsorption process. Intriguingly, TPG with large surface area exhibited excellent adsorption behaviors and good biocompatibility. The adsorbed materials were characterized by various methods including scanning electron microscopy (SEM), atomic force microscopy (AFM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR). And the effects of adsorption behavior of cyt c were discussed in detail. The results showed the adsorption behavior was dependent on the pH value and showed a high adsorption capacity as high as 1.414 × 104 mg/g and was friendly to normal cells (mouse fibroblast cell line, L929). In conclusion, we proposed the introduction of TPG as novel material and used the adsorption method to immobilize cyt c, which would provide a novel material and simple method for the enrichment of protein.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
These authors contributed equally to this work.
References
REFERENCES
- 4
- Cited by


