Article contents
New insights on the basicity of ZnAl–Zr hydrotalcites activated at low temperature and their application in transesterification of soybean oil
Published online by Cambridge University Press: 07 September 2018
Abstract
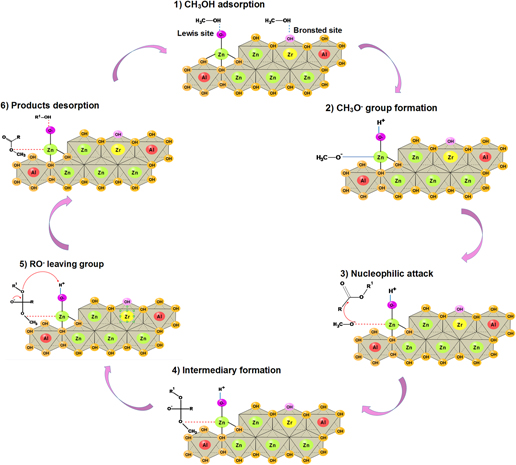
ZnAl–Zr(X) hydrotalcite-like materials were synthesized by co-precipitation using a Zn/Al molar ratio of 2 and Zr/Al(X) molar ratios of 0.0, 0.10, and 0.25. The effect of the activation temperature on the catalytic performance of these materials was analyzed, revealing that at relatively low temperature (200 °C), the collapse of the material structure is diminished, leading to FAME yields varying from 68 to 82%. This remarkable catalytic activity is related to the formation of hydrotalcite, zincite, and hydrozincite which in turn lead to the generation of Brönsted basic sites and Lewis acid–basic pairs. Incorporation of Zr+4 into the brucite-like structure of hydrotalcites enhances the basicity of ZnAl–Zr(X) catalysts, which correlates well with the increase in catalytic activity observed for these catalysts. The stability of the ZnAl–Zr(0.25) catalyst was further studied, showing insignificant deactivation after five subsequent reaction cycles. A simplified reaction scheme was proposed for the transesterification reaction over these materials.
- Type
- Article
- Information
- Journal of Materials Research , Volume 33 , Issue 21: Focus Issue: Catalytic Engineered Materials for Commercial and Industrial Energy Applications , 14 November 2018 , pp. 3614 - 3624
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 8
- Cited by


