Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Hou, Ping
Basu, S. N.
and
Sarin, V. K.
1999.
Nucleation mechanisms in chemically vapor-deposited mullite coatings on SiC.
Journal of Materials Research,
Vol. 14,
Issue. 7,
p.
2952.
Hou, Xiaoqiang
and
Kirkpatrick, R. James
2001.
Thermal Evolution of the Cl-−LiAl2 Layered Double Hydroxide: A Multinuclear MAS NMR and XRD Perspective.
Inorganic Chemistry,
Vol. 40,
Issue. 25,
p.
6397.
Yokoyama, Takushi
Ueda, Akira
Kato, Koichi
Mogi, Katsumi
and
Matsuo, Shorin
2002.
A Study of the Alumina–Silica Gel Adsorbent for the Removal of Silicic Acid from Geothermal Water: Increase in Adsorption Capacity of the Adsorbent due to Formation of Amorphous Aluminosilicate by Adsorption of Silicic Acid.
Journal of Colloid and Interface Science,
Vol. 252,
Issue. 1,
p.
1.
Miyazaki, Akane
Balint, Ioan
and
Nakano, Yoshio
2003.
Solid-liquid interfacial reaction of Zn2+ ions on the surface of amorphous aluminosilicates with various Al/Si ratios.
Geochimica et Cosmochimica Acta,
Vol. 67,
Issue. 20,
p.
3833.
Yokoyama, Takushi
Taguchi, Sachihiro
Motomura, Yoshinobu
Watanabe, Koichiro
Nakanishi, Tetsuya
Aramaki, Yoshinobu
and
Izawa, Eiji
2004.
The effect of aluminum on the biodeposition of silica in hot spring water: Chemical state of aluminum in siliceous deposits collected along the hot spring water stream of Steep Cone hot spring in Yellowstone National Park, USA.
Chemical Geology,
Vol. 212,
Issue. 3-4,
p.
329.
Nishida, Ikuko
Shimada, Yuuki
Saito, Tomoyuki
Okaue, Yoshihiro
and
Yokoyama, Takushi
2009.
Effect of aluminum on the deposition of silica scales in cooling water systems.
Journal of Colloid and Interface Science,
Vol. 335,
Issue. 1,
p.
18.
Bai, Shuqin
Naren, Gaowa
Noma, Hiroaki
Etou, Mayumi
Ohashi, Hironori
Fujino, Yasuhiro
Doi, Katsumi
Okaue, Yoshihiro
and
Yokoyama, Takushi
2012.
Silica deposition induced by isolated aluminum ions bound on chelate resin as a model compound of the surface of microbes.
Colloids and Surfaces B: Biointerfaces,
Vol. 95,
Issue. ,
p.
208.
Miyazaki, Akane
Numata, Mayumi
Etou, Mayumi
Yonezu, Kotaro
Balint, Ioan
and
Yokoyama, Takushi
2013.
Evidence for tetrahedral AlO4 formation induced by Zn2+ adsorption onto Al(OH)3 gel.
Colloids and Surfaces A: Physicochemical and Engineering Aspects,
Vol. 420,
Issue. ,
p.
115.
Tokoro, Chiharu
Suzuki, Shinya
Haraguchi, Daisuke
and
Izawa, Sayaka
2014.
Silicate Removal in Aluminum Hydroxide Co-Precipitation Process.
Materials,
Vol. 7,
Issue. 2,
p.
1084.
Otake, Tsubasa
Yokoyama, Takushi
Francisco, Paul C. M.
and
Watanabe, Koichiro
2015.
Effect of octahedrally coordinated aluminum ions on the uptake of Au(III) chloro-hydroxy complexes in Al-Si systems.
GEOCHEMICAL JOURNAL,
Vol. 49,
Issue. 4,
p.
343.
Nishida, Ikuko
2022.
Water-Formed Deposits.
p.
195.
Liu, Yu-Hsuan
Bootwala, Yousuf Z.
Jang, Gyoung Gug
Keum, Jong K.
Khor, Chia Miang
Hoek, Eric M. V.
Jassby, David
Tsouris, Costas
Mothersbaugh, Jim
and
Hatzell, Marta C.
2022.
Electroprecipitation Mechanism Enabling Silica and Hardness Removal through Aluminum-Based Electrocoagulation.
ACS ES&T Engineering,
Vol. 2,
Issue. 7,
p.
1200.
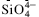 unit (no Si–O–Si bond), is important for the direct mullitization from AAS.
unit (no Si–O–Si bond), is important for the direct mullitization from AAS.