Published online by Cambridge University Press: 12 November 2018
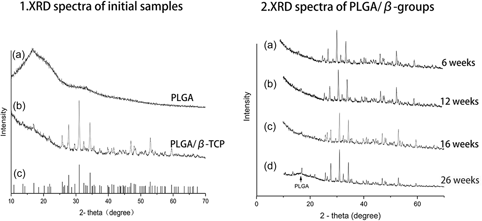
The aim of this study was to investigate the in vivo degradation mechanism and the mechanical properties of poly(lactide-co-glycolide)/beta-tricalcium phosphate (PLGA/β-TCP) composite anchors. Anchors composed of PLGA and β-TCP were implanted in the dorsal subcutaneous tissue of beagle dogs for 6, 12, 16, and 26 weeks. The degradation of the materials was evaluated by measuring the changes in thermal behavior, crystallinity, and mechanical properties. Scanning electron microscope (SEM) was used to observe the surface and longitudinal section of the material. The evaluation of mechanical strength retention and degradation properties suggest that the addition of β-TCP particles efficiently enhances their mechanical properties and thermal characteristics and delays their degradation rate. By analyzing the results of SEM, X-ray diffraction, and differential scanning calorimetry, we can infer that after 12 weeks, the connection between β-TCP and PLGA becomes less compact, which accelerates the decline of mechanical strength.
These authors contributed equally to this work.