Article contents
CuO–ZnO anchored on APS modified activated carbon as an enhanced catalyst for methanol synthesis—The role of ZnO
Published online by Cambridge University Press: 16 May 2018
Abstract
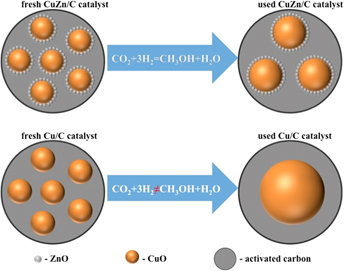
In this work, activated carbon was modified by ammonium persulfate and used as the catalyst support for CO2 hydrogenation to methanol. Then CuO and/or ZnO were loaded on the support by a facile wet-impregnation method. The obtained CuZn/C, Cu/C, and Zn/C catalysts were characterized by a series of characterization techniques including N2 physisorption, X-ray diffraction (XRD), X-ray photoelectron (XPS), and scanning and transmission electron microscopies (SEM and TEM). XRD and XPS results showed that ZnO affected the reduction of Cu2+. The TEM results showed that Cu particles were 14–18 nm for the fresh catalysts CuZn/C and Cu/C. ZnO particles were too small to be identified by TEM. The used catalysts CuZn/C and Cu/C had particle sizes of 10–25 nm and 50–60 nm, respectively. The enhanced methanol synthesis performance by ZnO could be ascribed to the morphology effect and slowing down the Cu particles sintering during the reactions.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 8
- Cited by



