Published online by Cambridge University Press: 12 December 2017
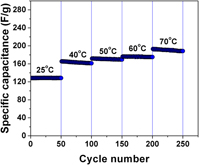
A hierarchical porous carbon with bimodal pore size distribution was synthesized using pyrolysis and controlled activation of polymer gel derived from furfuryl alcohol and phloroglucinol using a soft templating approach. Symmetric capacitors made using the synthesized carbon electrodes in neat 1-butyl 3-methylimidazolium tetrafluoroborate showed a specific capacitance of 141 F/g at 1 A/g when cycled between 0 and 3.8 V at room temperature. The capacitor also showed 90% capacitance retention over 5000 cycles. Equivalent circuit modeling of impedance spectra was done to monitor changes and provide microstructural details during cycling and temperature studies. Cyclic voltammetry showed the presence of specific adsorption of the electrolyte ions on the carbon electrode and this was in good agreement with strong dependence of specific capacitance on temperature. Such strong interaction along with the nature of electrical double layer formed in the hierarchical porous carbon results in widening the voltage stability window of the capacitor.
Contributing Editor: Yat Li