Article contents
Toward the design of interstitial nonmetals co-doping for Mg-based hydrides as hydrogen storage material
Published online by Cambridge University Press: 22 October 2018
Abstract
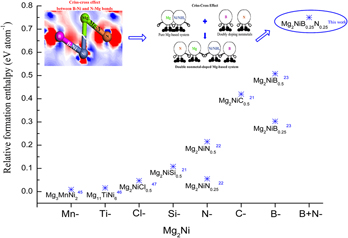
The strong interactions between Mg and Ni/NiH4 are attributed to harsh operating conditions and difficulties for H2 release, restricting the practical applications of the Mg-based hydrides. In this study, a new method of interstitial nonmetals co-doping was proposed to reduce the strong interactions. The calculation results showed that the method of interstitial nonmetals co-doping causes a more significant reduction in the thermal stability of Mg-based hydrides, as compared with the methods of either single transition metal or nonmetal doping. To determine the influence mechanism, a theoretical study was conducted based on the first-principles calculations. The computations demonstrated that the criss-cross action between B–Ni and N–Mg bonds weakens the bonding effects between Mg and Ni/NiH4. Besides, the mutual interactions between nonmetals and H atoms could weaken Ni–H bonding effects and stimulate the breaking of stable NiH4 clusters, thereby facilitating the release of H2 from the hydride.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 8
- Cited by


