Article contents
Relevant changes in the properties of Co(Ni)Mo/Al2O3 HDS catalysts modified by small amounts of SiO2
Published online by Cambridge University Press: 13 August 2018
Abstract
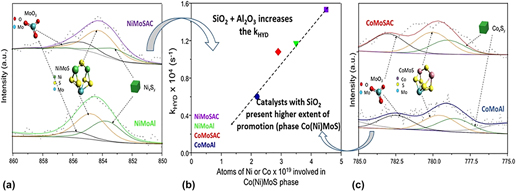
The changes in hydrodesulfurization activity, selectivity, dispersion, sulfidation, and extent of promotion of Co(Ni)Mo catalysts were investigated when the alumina support surface is modified by grafting 4 wt% silica. Adding SiO2 eliminates the most reactive hydroxyl groups on the alumina surface (IR band at 3775 cm−1) decreasing the possibility of generating tetrahedral Mo species difficult to sulfide in favor of octahedral ones capable of contributing to the sulfided active phase. The catalysts were evaluated in the hydrodesulfurization of 4,6-dimethyldibenzothiophene. Incorporating SiO2 to alumina increases the hydrogenation rate constant and therefore the global hydrodesulfurization rate of 4,6-dimethyldibenzothiophene and enhances the promotion of Mo by Co (or Ni). The global sulfidation of Ni is not affected by the addition of silica but the sulfidation of cobalt is significantly improved. The extent of promotion of the NiMo/Al2O3 and NiMo/SiO2/Al2O3 catalysts was greater than the one achieved in their Co-promoted counterparts.
- Type
- Article
- Information
- Journal of Materials Research , Volume 33 , Issue 21: Focus Issue: Catalytic Engineered Materials for Commercial and Industrial Energy Applications , 14 November 2018 , pp. 3570 - 3579
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 6
- Cited by


