Published online by Cambridge University Press: 21 June 2018
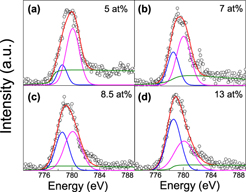
The valence states, the distribution of Co ions, and defect structures in the Co-doped ITO films with Co concentrations of 5–13 at.% were examined by X-ray absorption spectroscopy (XAS) at Co, K, and L-edges. The structural analyses and ab initio calculations reveal that the Co atoms are substantially incorporated into the ITO lattice and form cobalt–vacancy complexes, while partial formation of Co0 species is observed for all the films. The analyses of Co–K edge XAS reveal that the Co–O bond length RCo–O is shortened and the corresponding Debye–Waller factor (σ2) obviously increases with Co doping, implying the relaxation of oxygen environment around the substitutional Co ions. The qualitative fitting of Co L3-edge XAS further confirms the coexistence of Co0 and Co2+ in the films. The Co atoms mainly occupy the substitutional sites of In2O3 lattices with the metallic Co clusters being about 20–43 at.% for the 5, 7, and 8.5 at.% Co-doped ITO films. However, a significant fraction (∼57 at.%) of metallic Co clusters is found in the 13 at.% Co-doped ITO film.