Article contents
Improved performance of the functionalized nitinol as a prospective bone implant material
Published online by Cambridge University Press: 19 July 2018
Abstract
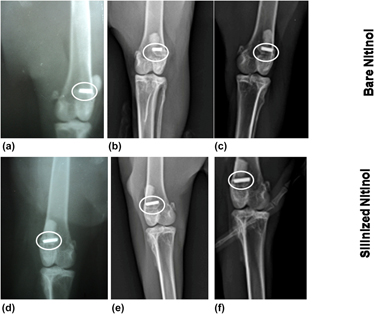
Nitinol, being a shape memory and super elastic alloy, is used in medical industry. Surface modification of nitinol helps to reduce the nickel ion leaching in physiological environment. The purpose of this study is to modify the nitinol surface by the silanization technique and to conduct a comparative investigation with the bare nitinol in the aspect of leaching of nickel ion, hemocompatibility, and in vivo animal response. X-ray photoelectron spectroscopy and energy dispersive X-ray spectroscopy studies confirmed the addition of organofunctional alkoxysilane molecules through the silanization process. The histological study showed the presence of adequate number of osteoblasts in silanized nitinol. The fluorochrome labeling study depicted more new bone formation (8 and 21% higher) in silanized nitinol specimens than bare one at one and three months postoperatively. Radiology and SEM study also proved the better performance of silanized samples. The cumulative in vivo results indicate its suitability as the potential bioimplant in various orthopedic surgical uses.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 6
- Cited by


