Published online by Cambridge University Press: 27 February 2018
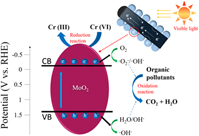
As an important member of semiconducting transition metal oxides, MoO2 nanomaterials have many advantages in optical and electrical applications. However, MoO2 itself has no significant photocatalytic performance possibly because of its inferior conductivity and strong recombination of photogenerated electron–hole pairs. Here, we propose a facile, one-step pyrolysis method to prepare a novel C fibers@MoO2 nanoparticles core–shell composite, where the oxidative MoO2 nanoparticles in situ grew on the surface of conducting C fibers. Due to the compositing of MoO2 and C fibers, during photocatalysis tests, the recombination of photogenerated electron–hole pairs was effectively inhibited, and the lifetime of the photogenerated carries was efficiently prolonged, finally significantly improving the solar-driven photocatalytic activity on degrading various organic and inorganic pollutants in water, such as methylene blue, rhodamine B, phenol, and potassium dichromate, showing the great potential for environmental remediation by degrading toxic industrial chemicals in waste water under sunlight. Moreover, the composite presented good stability in composition and structure during the repeated use and long-term storage. In addition, this one-step growth method is an easy-to-handle, environmentally friendly, and low-cost synthesis method for large-scale production.
Contributing Editor: Xiaobo Chen