INTRODUCTION
The spread of penicillin- and multidrug-resistant Streptococcus pneumoniae has become a worldwide problem in recent years, making it more difficult to treat invasive pneumococcal disease (IPD) [Reference Klugman1]. The risk of morbidity and mortality caused by IPD is particularly high for young children, the elderly, and immunocompromised individuals [Reference Klein2]. This risk has been increasing worldwide due to the development of resistance to antibiotics such as erythromycin, tetracycline and chloramphenicol, in addition to penicillin [Reference Felmingham3]. The non-susceptibility of bacterial isolates to antibiotics is considered to be strongly associated with their use in the patient community [Reference Goossens4].
In Europe, the prevalence of resistance to penicillin and macrolides is increasing mainly in the southern countries [Reference Bruinsma5], and the increase is associated with a small number of serotypes. A national surveillance study in Portugal indicated that penicillin non-susceptible strains of S. pneumoniae were responsible for 17·1% of cases of IPD in 1994–1998 [Reference Dias, Louro and Caniça6]. The most prevalent serogroups found then were 14, 23 and 9 [Reference Dias, Louro and Caniça6] but an increase in erythromycin-resistant isolates, predominantly serotype 14, was also documented in Portugal [Reference Dias and Caniça7]. The 7-valent pneumococcal conjugate vaccine protects against disease, and reduces the asymptomatic carriage of S. pneumoniae. Its generalized use also reduces the percentage of antibiotic-resistant strains with the serotypes most often implicated in invasive disease [Reference Whitney8]. This vaccine has been licensed for use in children aged <2 years in Portugal since 2001 and has been used voluntarily since then. We set out to evaluate the impact of antibiotic use and vaccination on antimicrobial resistance rates and explore the relationships between penicillin and erythromycin non-susceptible isolates and their serotype.
MATERIALS AND METHODS
Patients and bacterial isolates
The national laboratory surveillance study of pneumococcal infections in Portugal recovered 1331 S. pneumoniae isolates of invasive origin, between January 1994 and December 2004 (269 for the period 1994–1998, 491 for the period 1999–2001 and 571 for the period 2002–2004). A case of IPD was defined as disease in which a pneumococcus was isolated by culture from a normally sterile site (blood, CSF or pleural fluid) in a resident of the surveillance area. The isolates were collected in 24 hospitals throughout the country and one public health institution. The population covered by these centres in 2001 was 8 203 515 (79% of the Portuguese population), including 1 214 796 children (75% of Portuguese child population). There were 974 isolates from blood, 271 from CSF, 25 from both blood and CSF, 60 from pleural fluid, and one from both pleural fluid and blood. These were transported in trypticase soy broth (TSB; Oxoid, Basingstoke, UK) with 20% glycerol at −20°C to the Antibiotic Resistance Unit (ARU) at the National Institute of Health Dr. Ricardo Jorge in Lisbon. At ARU the identification and purity of strains were checked using standard microbiological methods and the strains stored at −80°C. The inclusion criteria for laboratory diagnosis were: non-repetitive and consecutive blood and/or CSF and/or pleural fluid sampling in individuals having symptoms compatible with IPD. Patients up to 18 years old were included in the paediatric population. Isolates from children aged <5 years accounted for 72% (240/332) of those from the paediatric population. The period up to 2000 was defined as the pre-vaccine period and between 2001 and 2004 was defined as the vaccine period.
Antimicrobial susceptibility testing
Minimum inhibitory concentrations (MIC) of penicillin (Wyeth Lederle Portugal, Algés), cefotaxime (Hoechst Marion Roussel, Mem Martins, Portugal), ceftriaxone (Roche Pharmaceuticals, Amadora, Portugal) and erythromycin (Abbott Laboratórios, Amadora, Portugal) were determined by an agar dilution method according to the conditions and interpretative criteria proposed by Clinical and Laboratory Standards Institute (CLSI), formerly NCCLS [9]. Intermediate- and high-level resistant strains to penicillin were analysed as a single group due to the low numbers (34/1331) of high-level resistant strains and were classified as non-susceptible.
Serotyping
Isolates were serotyped by dot-blot and/or the Quellung reaction using type-specific antiserum [Reference Fenoll10].
Antimicrobial and vaccine use data
National outpatient sale data were obtained, from 1994 to 2004, for the commonly used β-lactams and macrolides (amoxicillin, J01CA04; amoxicillin+clavulanic acid, J01CR02; flucloxacillin, J01CF05; cefatrizine, J01DB07; cefuroxime, J01DC02; cefaclor, J01DC04; cefixime, J01DD08; clarithromycin, J01FA09; erythromycin, J01FA01; azithromycin, J01FA10; and telithromycin, J01FA15). Data on anti-pneumococcal vaccine were obtained from International Medical Statistics (IMS Health). Antimicrobial usage data were grouped according to the Anatomic Therapeutic Chemical (ATC) classification and expressed as daily dose (DDD) units/1000 inhabitants daily (DID), as proposed by WHO [11]. Anti-pneumococcal vaccine use was expressed as annual units of pneumococcal heptavalent conjugate vaccine sold in Portugal. Linear associations between antimicrobial consumption, heptavalent vaccine use and rates of non-susceptibility were assessed by the Pearson correlation coefficient using a time lag of 0 or 1 year. The Mann–Whitney U test was performed to compare antimicrobial use between the period prior to (1994–2000) and after (2001–2004) the introduction of the vaccine.
Antimicrobial consumption, vaccination and antimicrobial resistance
The impact of antimicrobial and vaccine use on penicillin and erythromycin resistance rates was assessed using a linear regression method on data for isolates from children and adults for the period 1994–2004. Four models were adjusted for the following dependent variables: erythromycin non-susceptibility rate among children (EC); erythromycin non-susceptibility rate among adults (EA); penicillin non-susceptibility rate among children (PC) and penicillin non-susceptibility rate among adults (PA). To obtain the best-fitted set of independent variables for inclusion in each model, we performed a backward selection method on variables that reflected the use of antibiotics and vaccine which correlated positively with antimicrobial resistance rates (determined previously by the Pearson coefficient); these variables were also associated to the respective age of the population [Reference Falcão12]. When none or a single correlation was found to be associated with the dependent variables, the main antibiotics used were added to the input of the backward selection method as well as the anti-pneumococcal vaccine consumption. The reduced number of years and the correlation between the independent variables were the major limitations on the use of the multiple regression models in the study. The fit diagnosis was assessed by adjusted R 2 statistic and F ANOVA test. In each model, the regression coefficients associated with each independent variable were estimated by the ordinary least squares method [Reference Belsley13], and a P value for their significance and standard error were determined.
Preliminary analyses with the PC model suggested an association between the penicillin non-susceptibility rate among children and the following independent variables: amoxicillin, flucloxacillin, azithromycin and vaccine use. The latter two factors were removed from the equation corresponding to the PC model and new estimations of the values of the dependent variable were made based on the new equations obtained. All statistical procedures were undertaken with SPSS software, version 13.0 (SPSS Inc., Chicago, IL, USA). Two-sided P values <0·05 were considered as significant.
RESULTS
In total, 697 isolates of S. pneumoniae of invasive origin were recovered between January 1999 and December 2002 comprising 177 from children, of whom 105 were aged <2 years; 469 from adults of whom 182 were ⩾65 years; and 51 of unknown age.
Forty-five different serotypes were identified. Serotypes 14, 1, 23F, 6B, 7F, 19F, 9V, 3 and 6A (in descending order) accounted for 80% of the paediatric isolates comprising a total of 27 serotypes. Among adults, serotypes 3, 1, 14, 8, 4, 9V, 23F, 7F, 19F, 9N, 6B, 18C, 6A, 19A, 12F, 16F and 17F (in descending order) were responsible for 80% of infections.
Antimicrobial susceptibility and serotypes
The frequency of penicillin non-susceptible pneumococci from children aged <18 years was 36·4% (64/476) and from adults 15·8% (74/467). For children aged <2 years, 41% (43/105) yielded penicillin non-susceptible isolates of serotypes 14 (67·4%), 23F (18·6%), 6B (9·3%), 19A (2·3%) and 34 (2·3%); serotypes 9V and 19F were only isolated from children aged between 2 and 18 years (8/21) (Fig. 1a). In the elderly penicillin non-susceptible isolates (32/182) were predominantly serotype 14 (48·4%), 9V (22·6%) and 23F (9·7%) (Fig. 1b).
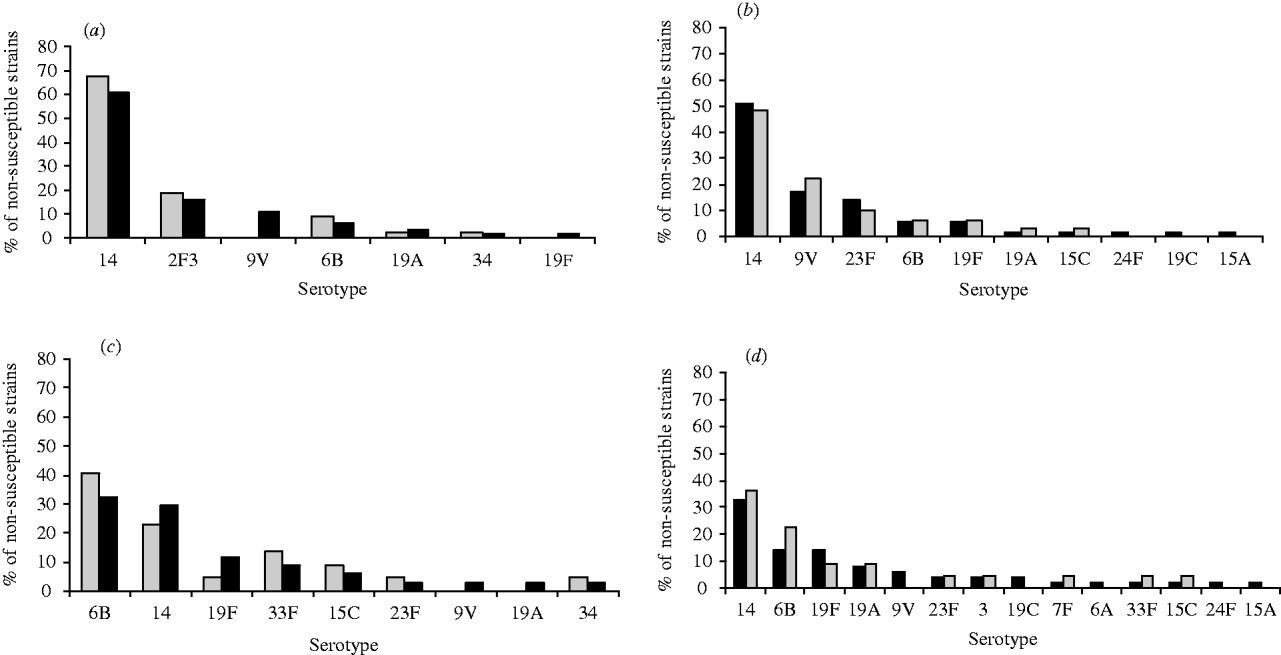
Fig. 1. Distribution of non-susceptible strains to penicillin and erythromycin by pneumococci serotypes isolated from invasive disease in Portugal between years 1999 and 2002. (a) Distribution of non-susceptible strains to penicillin by serotypes isolated from children aged <2 years (□) and <18 years (■). (b) Distribution of non-susceptible strains to penicillin by serotypes isolated from adults aged ⩾18 years (■) and ⩾65 years (□). (c) Distribution of non-susceptible strains to erythromycin by serotypes isolated from children aged <2 years (□) and <18 years (■). (d) Distribution of non-susceptible strains to erythromycin by serotypes isolated from adults aged ⩾18 years (■) and ⩾65 years (□).
A small number (8/105) of pneumococcal isolates from children aged <2 years were not susceptible to cefotaxime and ceftriaxone and only two isolates from the elderly were not susceptible to these agents; these isolates were also not susceptible to penicillin and were of serotypes 14, 9V and 6B. The frequency of erythromycin non-susceptible isolates from children aged <18 years was 19·2% (34/177) and among adults 10·7% (50/467); in the <2 years age group it accounted for 21% (22/105) and comprised serotypes 6B (40·9%), 14 (22·7%), 33F (13·6%), 15C (9·1%), 19F (4·5%), 23F (4·5%), and 34 (4·5%); serotypes 9V and 19A were only isolated from children aged between 2 and 18 years (Fig. 1c). Among cases aged ⩾65 years, erythromycin non-susceptible isolates accounted for 12·6% (23/182) and were mostly serotype 14 and 6B (Fig. 1d).
Vaccine coverage of drug-susceptible and resistant strains
The three conjugated vaccines (7-, 9-, 11-valent) have theoretical rates of coverage between 91·7% and 100% for penicillin non-susceptible isolates from children; a similarly high coverage is offered by the polysaccharide vaccine (95·8–100%). In the elderly, however, the theoretical rate was 93·5% for all conjugated vaccines, and 96·8% for the polysaccharide vaccine (Fig. 2a). For erythromycin non-susceptible isolates coverage of the conjugate vaccines fell to 63·6% (100% for children) and the highest coverage in the ⩾65 years age group was 81·8% for conjugated vaccine (the 11-valent formulation), and 96·8% for the polysaccharide vaccine (Fig. 2b). All vaccine types offered 100% coverage for cefotaxime and ceftriaxone non-susceptible isolates in both children and adults.
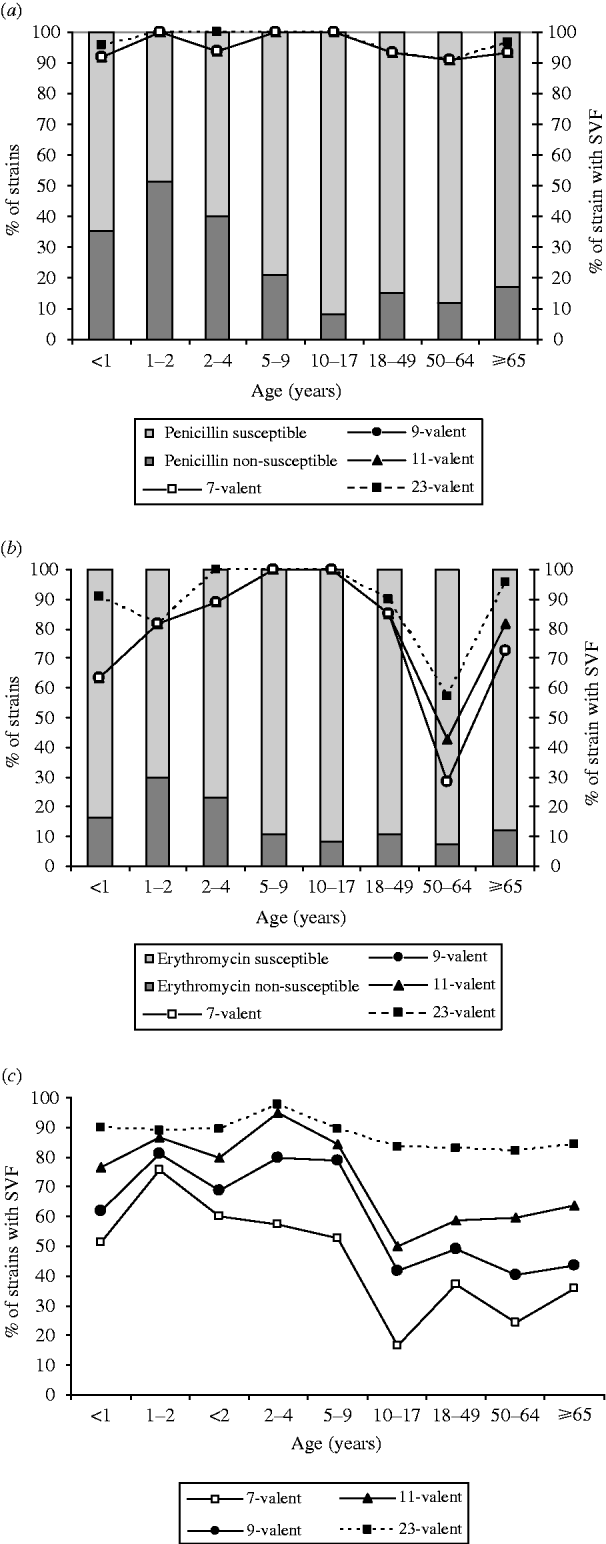
Fig. 2. Serotype vaccine coverage according to age group for penicillin and erythromycin non-susceptible strains. (a) Percentage of strains with serotypes included in the vaccine formulations (SVF), according to age group for penicillin non-susceptible strains. (b) Percentage of strains with SVF, according to age group for erythromycin non-susceptible strains. (c) Percentage of strains with SVF, according to age group for susceptible and non-susceptible strains.
Seven-, 9-, 11-valent vaccines gave theoretical coverage rates of 55·9%, 70·6% and 81·9% respectively for all S. pneumoniae isolated from children and the corresponding rates for the <2 years age group were 60·0%, 68·6% and 80·0% (Fig. 2c). The theoretical coverage of all formulations represented was higher for the 2–4 years age group, with the exception of the 7-valent vaccine. Children aged 10–17 years had the lowest coverage by the 7- (16·7%), 9- (41·7%) and 11-valent (50·0%) vaccines of all the subpopulations. Among adults the 7-, 9-, and 11-valent vaccines gave a theoretical coverage of 39·9%, 52·2% and 66·7%, respectively (Fig. 2c) with the 11-valent vaccine showing the highest rate, particularly for those aged >50 years, due to the higher frequency of serotype 3 in this group. Strains with serotypes included in the polysaccharide vaccine formulation made up 85·4% of the isolates from adults.
Antimicrobial use and resistance
Outpatient use of penicillin decreased between the period before and after the vaccine was introduced (P=0·04). The use of macrolides did not change significantly between the two periods (P=0·65). Amoxicillin and erythromycin were the main antibiotics with a decreased use in the vaccine period (P=0·04 and P<0·01, respectively). In contrast azithromycin use increased significantly (P<0·01), and clarithromycin use remained static (P=0·07) (Table 1). Telithromycin has only been on sale in Portugal since 2003 and its use increased in 2004.
Table 1. Antimicrobial and vaccine use in Portugal during 1994–2000 and 2001–2004
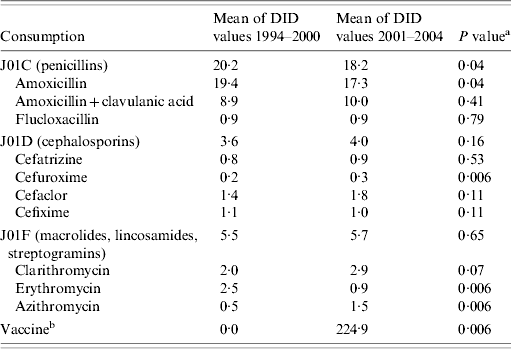
DID, Daily dose (DDD) units/1000 inhabitants daily.
a Mann–Whitney U test.
b Mean of 1000 pneumococcal heptavalent conjugate vaccine units sold.
The rate of non-susceptibility to erythromycin of pneumococcal isolates from children was found to be associated with macrolide use, mainly clarithromycin and azithromycin, as well as cephalosporins and amoxicillin+clavulanic acid (Table 2). In adults, the non-susceptibility rate to erythromycin was positively associated with prescribing of clarithromycin, azithromycin and cefuroxime, all with a time lag of 1 year; a positive association with the use of heptavalent conjugate vaccine was also found (Table 2). Penicillin non-susceptibility in children was linked only with amoxicillin use with a time lag of 1 year. Among adults, this rate was found to be positively associated to amoxicillin+clavulanic acid with a time lag of 1 year, and to cephalosporin, clarithromycin and azithromycin use (Table 2).
Table 2. Correlation between rates of susceptibility to penicillin and erythromycin in children and adults and antibiotic use
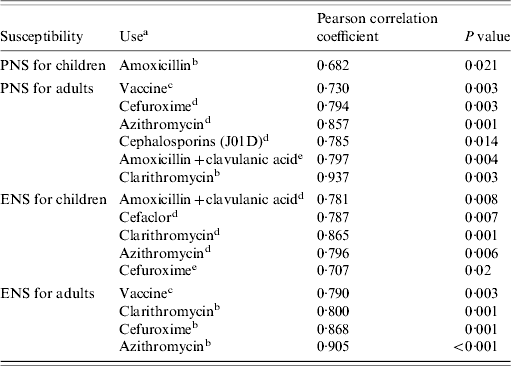
PNS, Penicillin non-susceptibility; ENS, erythromycin non-susceptibility.
a Only antibiotics which were associated with non-susceptibility are included.
b Exponential of antibiotic use with a time lag of 1 year.
c Heptavalent pneumococcal conjugate vaccine use.
d Antibiotic use.
e Antibiotic use with a time lag of 1 year.
Trends of penicillin and erythromycin resistance
The observed rates of penicillin non-susceptible pneumococci from children increased between 1994 (33·3%) and 2000 (56·8%), except for a decreased rate in 1998 and 1999 (pre-vaccine period). This rate decreased further to 21·3% in 2003 but increased to 40·0% in 2004 (Fig. 3a). No significant difference was found for observed rates of penicillin non-susceptible pneumococci between pre-vaccine (40·2%) and vaccine (31·7%) periods (P=0·125). The observed rate consistently increased in adults between 1994 (0·0%) and 2004 (17·0%) (Fig. 3b) and this was statistically significant for rates between the period prior to (11·0%) and after (17·0%) vaccine introduction (P=0·012).

Fig. 3. Observed annual rates (1994–2004) of antimicrobial non-susceptible pneumococci isolated from invasive disease in Portugal and respective linear regression models. (a) Observed (■) and predicted (–△–) annual rates of penicillin non-susceptible strains for children (PC). (b) Observed (■) and predicted (–△–) annual rates of penicillin non-susceptible strains for adults (PA). (c) Observed (■) and predicted (–△–) annual rates of erythromycin non-susceptible strains for children (EC). (d) Observed (■) and predicted (–△–) annual rates of erythromycin non-susceptible strains for adults (EA). (e) Scenarios for annual rates of penicillin non-susceptible strains for children: predicted (–△–) without vaccine (–×–), without azithromycin use (–◊–), without vaccine and azithromycin use (–+–).
Among children, erythromycin non-susceptible strains only emerged in 1997 and increased more consistently since 2000 (Fig. 3c); the rates for these strains did not show a significant increase before (16·7%) and during (22·0%) the vaccine period (P=0·260). However, in adults, the rates of erythromycin non-susceptible isolates increased from none in 1994 to 23·2% in 2004 (Fig. 3d) and showed a significant increase between in the vaccine period (P<0·001).
Model diagnosis
All models used in this study showed good adjustment with the observed data (Table 3). The irregularities observed in the PC model are probably associated with fluctuations in the consumption of several antibiotics. The EC model presented a lower adjustment (r=0·796±0·069) than the other models which could be due to the limited number of years that data were available. The year 1998 was excluded from analysis due to the unexpected high rate of non-susceptible isolates obtained that year. Isolates non-susceptible to erythromycin were first observed in Portugal in 1997, but showed only a defined trend since 2000. PA and EA models, showed good adjustments to the observed resistance rates (r=0·931±0·024 and r=0·939±0·015, respectively) (Table 3).
Table 3. Summary of statistical analyses of the four linear regression models used to describe non susceptibility rates
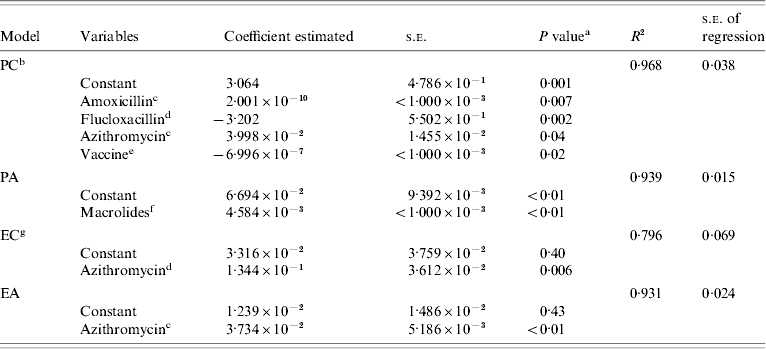
PC, Rates of penicillin non-susceptible strains for children; PA, rates of penicillin non-susceptible strains for adults; EC, rates of erythromycin non-susceptible strains for children; EA, rates of erythromycin non-susceptible strains for adults.
a t test for coefficient significance.
b Variables included in the model for backward selection: amoxicillin plus the main used antibiotics (i.e. clavulanic acid, flucloxacillin, clarithromycin, azithromycin) and vaccine consumption (see Materials and methods).
c Exponential of antibiotic use with a time lag of 1 year.
d Antibiotic use.
e Heptavalent pneumococcal conjugate vaccine use.
f Exponential of clarithromycin use with time lag of 1 year plus azithromycin use.
g Data for 1998 was excluded from this model due to the atypical values.
Parameters estimation and analysis
The EC and EA models suggest that the use of azithromycin was highly associated with macrolide resistance independent of the age group (Tables 2 and 3). The PC model suggests a link between the non-susceptibility of isolates and the use of amoxicillin, flucloxacillin and azithromycin. The detection of the effect of the introduction of the heptavalent vaccine in penicillin non-susceptibility among children is also of note (Table 3 and Fig. 3e). The PA model suggests that the use of clarithromycin and azithromycin were highly associated with penicillin non-susceptibility among adults with IPD (Table 3). Several scenarios were constructed based on the PC model. In the absence of vaccine introduction, non-susceptibility to penicillin was expected to achieve values greater than 55% in 2004 (Fig. 3e). In the presence of the pneumococcal conjugate vaccine and in the absence of azithromycin use, the penicillin non-susceptibility rate was expected to be reduced to 17% against the 40% observed in 2004. If no vaccine and azithromycin were used, the penicillin non-susceptibility rate was expected to be similar to that actually observed (with vaccine and azithromycin use) (Fig. 3e).
DISCUSSION
The introduction of the heptavalent pneumococcal conjugate vaccine was expected to reduce the burden caused by IPD and reduce the rates of antimicrobial non-susceptible isolates among pneumococcal strains. This vaccine was licensed in Portugal in 2001 and is currently recommended on a voluntary basis because it is not included in the national vaccination programme. The Portuguese Society of Paediatrics recommends the use of the conjugate vaccine in all children aged <2 years and in the population at risk for IPD [14]. In the north of Portugal, in 2001–2002, 54·5% of children born after 1999 were vaccinated against pneumococcal infection [Reference De Queirós15], and the use of this vaccine has been increasing from 2002 to 2005. It was estimated that vaccine coverage in 2004 was 65% among children aged <6 months who had received an average of 2·5 doses (data not shown).
Here, among children, we demonstrated a decrease (63%) in the rate of penicillin non-susceptible S. pneumoniae strains from 2000 (57%) to 2003 (21%), followed by an increase (40% in 2004). We were unable to show a significant difference in the rates of penicillin non-susceptible pneumococci strains between the pre-vaccine and vaccine period (P=0·125), although the observed early decrease may have been associated with vaccination. However, a decreased use of the main β-lactam agents was also evident in the same period (Table 1). This is consistent with the finding of Whitney and collaborators who also observed a reduction (35%) of penicillin non-susceptible strains from IPD in the United States, 2 years after the introduction of the vaccine [Reference Whitney8].
The PC model indicated that the non-susceptibility of isolates to penicillin among children correlated positively with the use of amoxicillin and azithromycin, and was negatively associated with the use of pneumococcal conjugate vaccine and flucloxacillin (Table 3). These antibiotics were the main β-lactams and macrolides used in Portugal, as found in this study and others [Reference Goossens4, Reference Dias and Caniça7, Reference Falcão12]. The PC model also suggests that if the vaccine had not been introduced in Portugal, non-susceptibility to penicillin was expected to achieve values greater than 55% in 2004 (Fig. 3e). This might mean that the pneumococcal conjugate vaccine had potentially reduced by more than 15% the rate of penicillin non-susceptibility. However, our results also suggest that azithromycin use in the studied population limited the efficiency of the pneumococcal conjugate vaccine. Indeed, if pneumococcal conjugant vaccine was used and azithromycin was not used, penicillin non-susceptibility was predicted to be reduced to 17% in 2004 (Fig. 3e), a similar reduction was experienced by Whitney after the introduction of anti-pneumococcal conjugate vaccine in the United States [Reference Whitney8]. Conversely, if no vaccine or azithromycin were used, the penicillin non-susceptibility rate was predicted to be similar to that observed (Fig. 3e).
The increase of penicillin non-susceptibility among isolates was due to cross-resistance to macrolides and not to changes in the consumption of β-lactams (Table 1). Several studies have shown cross-resistance to be responsible for the emergence of resistance to other antibiotics. Amoxicillin use significantly increased the number of amoxicillin+erythromycin resistant isolates [Reference Ready16]. However, other antibiotics (e.g. cephalosporins, macrolides and co-trimoxazole) can be equally or more important than aminopenicillins in promoting the selection of penicillin non-susceptible pneumococci [Reference Dancer17], and selection by cephalosporins can occur at higher frequencies than selection by amoxicillin [Reference Canet and Garau18]. The use of macrolides has been reported to be a more effective selector of strains non-susceptible to macrolides+β-lactams than the use of aminopenicillins [Reference Arason19, Reference Baquero20], and azithromycin use has been linked to macrolide and other resistances in pneumococci [Reference Dias and Caniça7, Reference Barkai21]. As observed recently in Portugal [Reference Dias, Louro and Caniça6], there has also been an increase of penicillin resistance associated with macrolide resistance elsewhere [Reference Barkai21, Reference Bronzwaer22]. These results are consistent with the PC and PA models (Fig. 3 and Table 3). Indeed, the high potential of azithromycin to select resistant, multiresistant, and co-resistant strains, may be due to its long half-life (<72 h) [Reference Nightingale23], and its very slow elimination resulting in subinhibitory concentrations in the tissues [Reference Kastner and Guggenbichler24].
In this study amoxicillin+clavulanic acid use was found to be associated with non-susceptibility to penicillin and erythromycin (Table 1), however, our models showed that their impact is reduced in penicillin non-susceptible rates (Table 3). Amoxicillin+clavulanic acid has been described to eradicate or suppress all pneumococci susceptible to penicillin and has good activity against strains with intermediate and/or full resistance to penicillin [Reference Ghaffar25]. The latter study also reported that azithromycin (in children with acute otitis media) was able to clear 33% of susceptible pneumococcal strains, but none of the azithromycin non-susceptible strains tested was eliminated [Reference Ghaffar25]. Azithromycin has also been associated with the selection of a higher number of resistant clones than amoxicillin-clavulanate [Reference Kosowska-Shick26]. These findings are consistent with the suggested impact of these antibiotics by the models used.
The largest groups of S. pneumoniae strains not susceptible to penicillin and erythromycin, in our population, were of serotypes covered by the 7-valent vaccine. Similar to previous studies [Reference Whitney8], our results suggest that the vaccine covers almost all non-susceptible serotypes. However, resistance was also found in serotypes 15C, 19A, and 34, which are absent from the vaccine. This may be a concern after vaccination, as Lipsitch pointed out, because serotype replacement is expected in an ecosystem where many serotypes are present [Reference Lipsitch27]. Studies of S. pneumoniae isolated in day-care centres indicate that the 7-valent vaccine can reduce the carriage of resistant isolates [Reference Dagan28]. A Portuguese study showed a decrease in vaccine serotypes, but an increase of non-vaccine serotypes among drug-resistant S. pneumoniae isolated from children attending day-care centres [Reference Frazão29], this is supported by Temime et al. [Reference Temime, Guillemot and Boelle30] underlining that serotype replacement constitutes a real threat. Indeed, the replacement effect involving penicillin non-susceptible strains usually isolated from carriers might accelerate in the coming years among strains isolated from meningitis, as the nasopharynx is an important reservoir of such strains [Reference Bogaert, De Groot and Hermans31] and the selection pressure caused by antibiotics is continuous [Reference Cars, Molstad and Melander32].
We found similar rates of vaccine coverage of penicillin non-susceptible strains to those reported in other European countries and in the United States [Reference Whitney8, Reference Reinert33]. The theoretical overall coverage conferred by the pneumococcal conjugate vaccine in Portugal is similar to that in some other European countries [Reference Reinert33], but lower than the coverage shown in the United States [Reference Whitney8]. As noted previously, we found penicillin non-susceptible strains to also be non-susceptible to cefotaxime and ceftriaxone [Reference Caniça34], but specifically among children aged <2 years there are high rates of resistance (7·6%) to these agents, which are the most widely used for empirical treatment of meningitis caused by S. pneumoniae [35]. Thus, the replacement effect to non-vaccine serotypes that occurs among penicillin non-susceptible strains can also contribute to a decrease in the vaccine coverage of cefotaxime and ceftriaxone non-susceptible strains in the future.
Since the introduction of azithromycin in Portugal in 1996 extensive changes among circulating clones of pneumococci have been observed [Reference Dias and Caniça7]. As a consequence, erythromycin non-susceptible rates among isolates from children between 1996 and 1999 were atypical, as they were higher than the observed trend in the following years; thus, the EC model did not fit so well. However, outside of this period, we did not observe marked differences in trends of erythromycin non-susceptible strains during the pre-vaccine and vaccine periods, as suggested by the EC model. This indicates that the introduction of the conjugate vaccine into Portugal did not significantly decrease the incidence of erythromycin non-susceptible strains due to the increased use of azithromycin (Fig. 3, Tables 1 and 3).
Our results illustrate that the use of macrolides class has been stable in the last years, but there has been a substitution of older macrolides mainly by azithromycin. No cross-resistance between macrolides (erythromycin) and telithromycin has been shown [Reference Walsh36], but recently the first telithromycin-resistant and otherwise multiresistant isolate of serotype 15A (non-vaccine serotype) was reported from Germany [Reference Reinert, van der Linden and Al-Lahham37]. In Portugal, extensive use of telithromycin in a population who have received the vaccine could lead to the emergence of this clone, with serious implications for resistance levels to penicillin and macrolides due to cross-resistance.
The changes in penicillin susceptibility in S. pneumoniae are mainly clonal [Reference Caniça38], but for erythromycin susceptibility the selective pressure causes greater oscillations and adaptations within the bacterial population, due to the nature of the resistance and its dissemination [Reference Pozzi39]. Hence, there is a greater diversity of serotypes among erythromycin non-susceptible pneumococci than among penicillin non-susceptible strains which cannot be explained solely by drug use. Indeed, the greater diversity of non-susceptible strains in addition to the selective pressure caused by vaccine and antibiotic consumption could enhance horizontal transfer of resistance elements among non-vaccine serotypes and/or capsular switching among non-susceptible vaccine serotypes. Thus, replacement among erythromycin non-susceptible strains seems to involve a rapid turnover, and this may have serious consequences for public health.
In conclusion, linear regression models were used to assess the role of antimicrobial and vaccine use in the rates of penicillin and erythromycin resistance among pneumococci. The models did not allow a mechanistic interpretation of the antibiotic resistance phenomena, but they provided very useful information on main factors involved in the selection of penicillin- and erythromycin-resistant strains: a complex context where multiple antimicrobial and vaccine use are present. Moreover, the suggestions obtained by the models fitted well with phenotypic observations. It is theoretically possible that the observed trends of antibiotic resistance in Portugal may be reversed by the adequate use of existing and new antibiotics, contributing to control of IPD. The reduced number of years and the correlation between the independent variables were the major limitations on the use of the multiple regression models in the study.
ACKNOWLEDGEMENTS
We thank Deolinda Louro for expert technical assistance, Luis Oliveira for invaluable help and suggestions regarding the linear regression models and Baltazar Nunes for his critical reading and suggestions at a statistical level. We acknowledge IMS Health, in Portugal, for data of antimicrobial consumption. Serotyping was partially supported by a grant from Wyeth Lederle, Portugal, and the National Institute of Health Dr. Ricardo Jorge funded the remaining study. R.D. was supported by a ‘NIH Scientific Research Dr. Ricardo-Jorge’ grant. We also thank the following hospital microbiology laboratories, participants of the national surveillance of pneumococcal infections through European Antimicrobial Resistance Surveillance System (EARSS) and Antimicrobial Resistance Surveillance Programme in Portugal (ARSIP) groups, for providing strains and data: Hospital S. António, Porto (S. Microbiologia); Hospital D. Estefânia, Lisboa (R. Barros); Hospital Eduardo Santos Silva, V. Nova de Gaia (C. Magalhães); Hospital S. Maria, Lisboa (M. J. Salgado); Hospital Garcia da Orta, Almada (M. Pinto, J. Diogo); Centro Hospitalar de Coimbra, Coimbra (L. Albuquerque); Hospital Especializado de Crianças Maria Pia, Porto (F. Teixeira); Hospital S. Francisco Xavier, Lisboa (F. Martins); Hospital Fernando da Fonseca, Amadora (L. Sancho); Instituto Nacional de Saúde, Porto (L. Bacteriologia); Hospital da Universidade de Coimbra, Coimbra (G. Ribeiro); Hospital S. José, Lisboa (O. Spencer); Hospital Curry Cabral, Lisboa (J. Serra); Hospital S. Teotónio, Viseu (J. Ribeiro); Hospital José Joaquim Fernandes, Beja (R. Bento); Hospital Conde Castro Guimarães, Cascais (A. Coutinho); Hospital N. S. Rosário, Barreiro (A. Jesus); Centro Hospitalar, Funchal (T. Afonso); IPO, Porto (I. Romero); Hospital de S. Luzia, Viana do Castelo (A. Santos); Centro Hospitalar de V. Real, Vila Real (A. Castro); Hospital Reynaldo dos Santos, V. Franca de Xira (M. Vasconcelos); Hospital SAMS, Lisboa (L. Cabral).
DECLARATION OF INTEREST
None.








