Staphylococcus aureus has long been recognized as one of the most common causes of both endemic and epidemic infections acquired in hospitals, resulting in substantial morbidity and mortality [Reference Kluytmans, van Belkum and Verbrugh1, Reference Klein, Smith and Laxminarayan2]. This is exacerbated by the increasing appearance of multidrug-resistant strains especially those with resistance to methicillin (MRSA) which represent a serious clinical threat and therapeutic challenge not only to hospitalized patients but also to adults and children in the community [Reference Lowy3, Reference Lu4]. The epidemiology of MRSA has changed radically in recent years with the definition of two groups of strain populations, hospital acquired and community acquired. The former is mostly associated with infections in the healthcare setting while the latter has been increasingly reported in healthy persons living in the community and is not associated with traditional MRSA risk factors (i.e. contact with healthcare facilities, previous antimicrobial therapy). These infections most often present as skin and soft tissue infections but occasionally as primary pneumonia with high morbidity and mortality [Reference Gorwitz5].
Carriage of S. aureus in the nasal passages appears to play a key role in the epidemiology and pathogenesis of infection [Reference Kluytmans, van Belkum and Verbrugh1]. Colonizing strains may serve as endogenous reservoirs for overt clinical infections or may spread to other patients [Reference Eiff6]. Data on the carriage rate and antibiotic susceptibility pattern of S. aureus strains in the Lebanese community are scarce [Reference Araj7]. This prospective study was therefore undertaken to determine the current carriage rate of S. aureus [methicillin-susceptible S. aureus and MRSA] in school and university students and employees working in these institutions. The possible contributing risk factors involved in colonization were also assessed.
In total 500 nasal swab samples were collected from school and university students and employees in the cities of Beirut and Sidon between September 2006 and March 2007. All participants were interviewed and a questionnaire was completed detailing name, age, gender, previous or current antibiotic treatment, history of hospital admission and previous surgery, contact with healthcare workers (HCWs), needle injections, daily nasal wash with water, use of nasal medication sprays, having acne problems, smoking, and suffering from asthma.
Sterile cotton swabs with Stuart's transport medium (Deltalab, Spain) were used for collection and transport of specimens. Sampling was performed by rotating a swab pre-wetted with sterile saline in the nares of each participant. The swabs were transported at 4°C to the microbiology laboratory for culture, most within 4 h of collection. They were streaked onto mannitol salt agar (MSA; Oxoid, UK), incubated at 35°C and examined for growth after 24–48 h. Mannitol-fermenting colonies were subcultured to nutrient agar and incubated at 35°C for 24 h. Isolates were identified as S. aureus by Gram stain, production of catalase, DNase, and coagulase in tube tests.
Susceptibility testing was performed by the disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guidelines [8] with S. aureus (ATCC 25923) as a control strain. Isolates were screened for susceptibility to amoxicillin/clavulanic acid, ampicillin, cefoxitin, cephalothin, ciprofloxacin, clindamicin, erythromycin, gentamicin, nitrofurantoin, oxacillin, penicillin, rifampicin, trimethoprim/sulfamethoxazole, tetracycline, fusidic acid, mupirocin, and vancomycin. Interpretation of zone inhibition diameters was according to CLSI guidelines except for fusidic acid [Reference Andrews9] and mupirocin [Reference Udo, Jacob and Mathew10]. Isolates resistant to 1 μg oxacillin and 30 μg cefoxitin were classified as MRSA [8]. Induced clindamycin resistance was inferred by the appearance of the inhibition zone between erythromycin and clindamycin disks [8]. Isolates were screened for resistance to vancomycin on brain heart infusion agar containing 6 μg/ml vancomycin [8].
Data were analysed using Yates corrected χ2 test. P values <0·05 were taken as significant. Statistical analysis was performed using Minitab and Epi-Info 3.4.3 soft ware (Centers for Disease Control and Prevention and World Health Organization).
Of the 500 participants in this study, 277 (55·4%) were females and 223 (44·6%) were males ranging in age from 6 to 65 years. Overall, 192 individuals (38·4%, 95% CI 34·1–42·8) were colonized by S. aureus, of which eight isolates (4·2%) were confirmed as MRSA. The age of individuals was significantly associated with S. aureus carriage (P<0·001). Subjects aged 6–10 years had a higher carriage rate (57·1%, 95% CI 48·0–65·9), compared to 11–17 years (34·9%, 95% CI 28·1–42·3), 18–25 years (24·8%, 95% CI 17·8–32·9), 26–40 years (37·0%, 95% CI 19·4–57·6), and 41–65 years (45·8%, 95% CI 25·6–67·2), respectively. The higher colonization rate therefore extended to those of school age (aged 6–17 years), declined during university attendance, and increased again in later life (aged 18–65 years) (43·9% vs. 29·3%, P=0·001). Males showed significantly higher (P=0·02) S. aureus colonization rates (43·5%) than females (34·3%).
The overall carriage rate for S. aureus of 38·4% exceeded rates reported from several other countries such as Saudi Arabia (20·2%) [Reference Panhotra, Saxena and Al Mulhim11], Italy (25·9%) [Reference Zanelli12] and the USA (31·6%) [Reference Graham, Lin and Larson13]. Moreover, the current carriage rate is higher than the 20% rate found in a survey of Lebanese individuals in the community in 1993 [Reference Araj7]. The latter study reported a MRSA colonization rate of 0·3% compared to the 1·6% rate found in the present study.
Children have been reported to have increasing rates of infection with S. aureus and MRSA [Reference Gorwitz5]. In Lebanon and the wider region, the effect of age on colonization rate had not been previously addressed. Reports from other countries also cite high rates of S. aureus colonization in children, e.g. 35% in children aged 3–11 years in Italy [Reference Zanelli12], and 28·4% in the 4–6 years age group in Turkey [Reference Ciftci14]. The significant difference in carriage between the school community subjects and the adult general population may be due to the closed school community environment facilitating the spread of bacteria.
Table 1 shows that the risk factors for a high carriage rate of S. aureus were: use of antibiotics in the last 6 weeks, hospital admittance in the last year, regular contact with HCWs, using needle injections, or having asthma. Lower colonization rates were significantly associated with participants having nasal wash with water more than twice daily, regular use of nasal sprays, and the presence of acne (P⩽0·02). Smoking and previous surgical operations did not appear to be associated with S. aureus nasal colonization.
Table 1. Factors associated with Staphylococcus aureus colonization in a Lebanese community
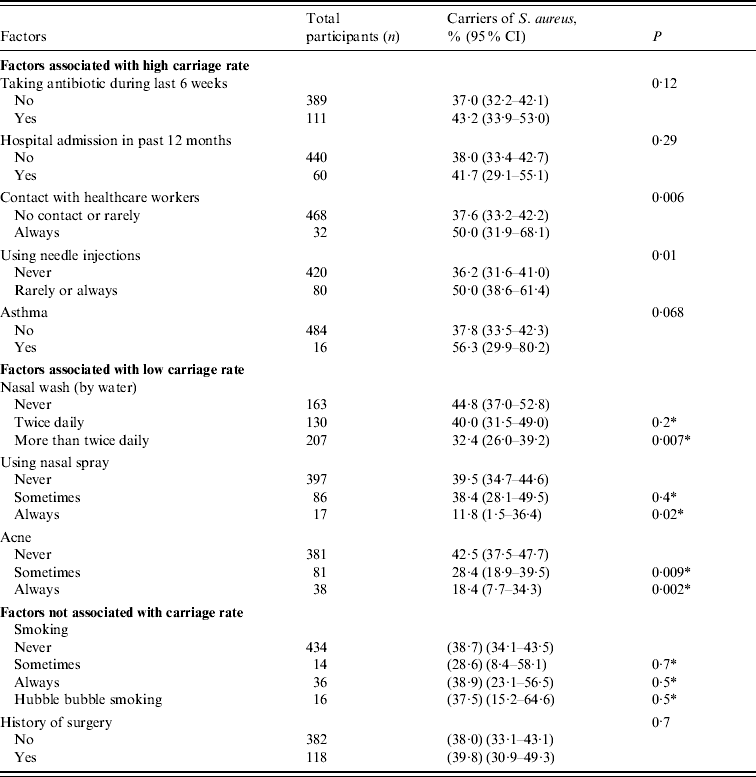
CI, Confidence interval.
* Comparing the corresponding factor to the never factor.
The higher colonization rate in individuals in regular contact with HCWs may be explained by contact of the latter with patients, colonized or infected by S. aureus, who as a result have higher rates of nasal carriage [Reference Cesur and Cokca15]. This reinforces the need for HCWs to be regularly screened for MRSA and, if positive, undergo decontamination to prevent further transmission of these organisms to others in the general population and the hospital. Impaired immune function or corticosteroid use by asthmatics, and/or their more frequent contact with HCWs could be an explanation for increased carriage in this group.
To our knowledge, this is the first time that nasal washing with water has been shown to affect S. aureus nasal colonization. Water may decrease the adherence of S. aureus by interrupting the physicochemical forces including hydrophobic interaction needed for bacterial adherence [Reference Beachy16] resulting in the elimination of the organism. Similarly, nasal medication sprays may mechanically decrease adherence of S. aureus to nasal mucosa but the contribution of the vehicle or medication to lower carriage rates should be considered. The correlation of acne with low carriage rates is an interesting finding and may be related to competition between S. aureus and Propionibacterium spp., which are frequent colonists of the anterior nares of individuals, as increased staphylococcal numbers have been reported to be associated with a parallel reduction of the number of Propionibacterium [Reference Coates17]. Tobacco smoking appears to have a noticeable effect on the microbial ecology of the nose [Reference Durmaz18]. Generally, smokers harboured a greater number of S. aureus (judged by the primary growth on MSA) but no statistical association was confirmed for their colonization rates over non- smokers.
Apart from a very low rate of susceptibility (5·7%) to penicillin and ampicillin, most isolates were susceptible (⩾94%) to all other antimicrobials tested except for erythromycin (82·8%), clindamycin (86·5%), fusidic acid (91·1%), and tetracycline (92·2%); no resistance to trimethoprim/sulfamethoxazole, mupirocin and vancomycin was observed. In addition, all MRSA isolates were susceptible to gentamicin, ciprofloxacin and nitrofurantoin.
In conclusion, this study sheds light on S. aureus carriage rates in a Lebanese community and establishes a basis for future surveillance of carriage. Despite a relatively high overall rate compared to some other countries, the level of MRSA carriage remains very low. Further investigations are needed to evaluate the effectiveness of nasal wash with water and use of nasal sprays in reducing colonization rates by S. aureus especially in HCWs. This might be useful as a hygiene measure in tandem with effective hand washing. Finally, the unexpected relationship between acne and decreased S. aureus carriage rate and the possible influence of P. acnes warrants further investigation.
ACKNOWLEDGEMENTS
We thank Professor Mahmoud Gabr, Dean of the Faculty of Science at Alexandria University, for help with the statistical analysis. Thanks are also due to all school principals, and Beirut Arab University staff and students for their valuable cooperation.
DECLARATION OF INTEREST
None.



