INTRODUCTION
Legionnaires' disease (LD) is an atypical pneumonia caused by bacteria of the genus Legionella [Reference Fraser1, Reference McDade2], which is an environmental microorganism found in soil and water. LD is a well described aetiology of community-acquired bacterial pneumonia in adults [Reference Huang3–Reference Woodhead6], with a high case-fatality ratio (CFR) (10–30%) [Reference Gacouin7–Reference Muder, Yu and Fang9]. Inhalation of aerosolized water containing Legionella is the primary mode of acquiring LD. Knowledge about risk factors derives mainly from outbreak studies [Reference Fraser1, Reference Nguyen10, Reference Monforte11], case series [Reference England and Fraser12–Reference Marston, Lipman and Breiman14] or studies comparing cases of pneumonia due to Legionella with cases due to other bacteria [Reference Carratala15]. Advanced age, smoking, immunosuppressive medication, chronic underlying illness such as end-stage renal disease, chronic lung disease and cancer were identified as factors that increase the risk of being infected after exposure. Nevertheless, little is known about the specific risk factors for sporadic, community-acquired LD, which account for more than 50% of the cases notified every year in France [Reference Campese16] and in other developed countries [Reference Ricketts and Joseph17]. The number of published studies conducted on risk factors for community-acquired LD is limited [Reference den Boer, Nijhof and Friesema18–Reference Straus20], and conclusions are difficult to draw because of different study designs and methodology. Furthermore, a recent paper showed that sporadic cases were more severe and more often associated with a poor outcome when compared to outbreak cases [Reference Sopena21], thus reinforcing the need for early detection of cases. The purpose of this study was to identify risk factors for sporadic, community-acquired LD related to the hosts, their activities and the environment.
MATERIALS AND METHODS
Source of information
In France, mandatory notification of LD was established in 1987. Physicians and microbiologists are required to notify confirmed and probable cases of LD to the district health officers who in turn notify the national health authority (Institut de Veille Sanitaire). The number of cases notified has increased over the years, especially since the introduction of urinary antigen tests in 1997. The sensitivity of the system also increased over the same period [Reference Nardone22]. In 2002, 1021 cases of LD were notified, corresponding to an estimated incidence of 1·7/100 000 inhabitants [Reference Campese23].
Study design
We used a prospective matched case-control study to assess the relationship between the occurrence of LD and outcome variables.
Case definition
A confirmed case of LD was defined as a person who had a radiographically confirmed pneumonia and laboratory evidence of infection with Legionella [i.e. isolation of Legionella from respiratory secretions, detection of Legionella pneumophila sg 1 antigens in urine, or a minimum fourfold rise (to ⩾128) in antibody titres to Legionella in convalescent serum compared with acute serum]. A sporadic case of LD was defined as a case that was not part of an identified outbreak. Patients known to have been hospitalized for at least 1 day during the 10 days prior to the onset of the disease or to have lived in a medicalized nursing home were assumed to have infections that were possibly nosocomial and were excluded from this study.
Selection of respondents
Cases were identified through the mandatory notification system. All were asked by local health officers to participate. Cases who agreed to participate completed a consent form and were interviewed by local health officers through a phone standardized questionnaire within 15 days of the onset of the disease. If the case was unable to answer (e.g. because of death, or hospitalization in an intensive care unit requiring mechanical ventilation), a relative was asked to complete the consent form and to answer the questionnaire. Controls were selected by the general practitioners of the cases among their patients and interviewed by phone by the data manager of the study (at the national level) using the same questionnaire as for cases. One control per case was matched according to sex, age (within 10 years for cases aged <65 years, and within 5 years for cases aged ⩾65 years), underlying conditions (absence of underlying illness; chronic disorders such as chronic pulmonary or renal disease; immunosuppressive conditions defined as the use of corticosteroid or receipt of chemotherapy, or AIDS) and location of residence (within 5 km). Controls with a history of pneumonia during the month prior to the interview were excluded.
Questions related to the 10-day period prior to the onset of the disease for cases and to the 10-day period prior to the interview for controls. In order to minimize recall bias and in order to avoid differing seasonal exposure for cases and their controls, controls were interviewed during the month following the onset of symptoms of their matched case. Ethical approval for this study was obtained from the Commission Nationale de l'Informatique et des Libertés.
Assuming an exposure rate varying from 10% to 70% among control subjects, a two-tailed significance level of 5%, and a power level of 80%, the enrolment of 600 cases and 600 controls was expected to permit detection of a minimal odds ratio (OR) of 1·5.
Cases meeting inclusion criteria and with onset of symptoms from 1 September 2002 to 31 September 2004 were included.
Variables studied
Variables were collected in order to document the individual health status (oxygen use, smoking status, alcohol intake, etc.), the environment (home setting, origin of drinking water, type and age of water heaters, etc.) and the professional and leisure activities (profession, history of travel and type of accommodation, gardening, aquatic sport, outdoor activities, exposure to water aerosols, etc.).
Seven categories were defined to characterize cases and controls according to their smoking status: non-smokers, ex-smokers (number of years of exposure ⩽20 years or >20 years) and current smokers (number of years of exposure ⩽20 years or >20 years, and number of cigarettes smoked ⩽20 per day or >20 per day) [Reference Almirall24–Reference Greig26]. Alcohol abuse was defined as the consumption of more than three units of alcohol per day for a man, and more than two units for a woman, considering a higher risk of LD for heavy drinkers as previously described [Reference den Boer, Nijhof and Friesema18, Reference Storch19]. Some variables previously identified as increasing the risk of being infected were also collected, such as living near excavation sites [Reference Storch19] or recent residential plumbing repairs [Reference Straus20].
Statistical analysis
Data were entered into Epi-Info, version 6 (Centers for Disease Control and Prevention, Atlanta, GA, USA). Crude association was determined by estimating the OR and its 95% confidence interval (CI). This was done by univariate conditional logistic regression, to account for the matched design. OR significance was assessed by the Wald test. A conditional logistic regression analysis, using EGRET® software (Egret for Windows 2.0.2, Cytel Software Corp., Cambridge, MA, USA) was performed, which included variables identified by the univariate analysis as being associated with LD, using a conservative threshold of P⩽0·20. A backward-step selection procedure was used to obtain the best model, and adjusted ORs (aORs) were reported. In order to limit the potential bias which may be associated with undocumented confounding variables, the results were adjusted for socio-professional categories. Interactions in the final model were tested.
In order to assess whether biases may have been introduced in selecting cases, the cases of LD included in the analysis were compared to cases that were registered in the national LD database but not included because of refusal, delay in responding, etc.
RESULTS
Between 1 September 2002 and 31 September 2004, 602 cases and 602 controls were recruited and interviewed. Matching criteria were not consistent for 56 pairs, and the remaining 546 matching pairs were finally included in the analysis. The 56 excluded cases did not differ significantly from the 546 included cases in terms of age, sex and underlying conditions.
For the 546 cases, the mean age was 57 years and the male:female sex ratio was 3·6 (428 men and 118 women). Underlying illness was identified in 29% of cases (22·5% with chronic disorders and 6·5% with immunosuppression). Nineteen cases had died before the interview (3·5%) and relatives were interviewed.
Urinary antigen detection of L. pneumophila sg 1 was positive for 510 cases (93·4%), L. pneumophila was isolated from the sputum of 73 cases (13·4%) and seroconversion was detected in 61 (11·2%). L. pneumophila sg 1 accounted for 94·5% of the isolates.
During the same period of time, 1155 sporadic community-acquired cases of LD were notified through the mandatory notification system but not interviewed. These patients did not differ from those included by sex but were older (mean age 59·5 vs. 57 years, P<0·01) and the CFR was significantly higher (11·4% vs. 3·5%, P<0·01).
Univariate analysis
Analysis of host-related factors indicated that current tobacco smoking was strongly associated with LD (global Wald statistics, P<0·001) as was excessive alcohol intake (aOR 1·83, 95% CI 1·28–2·61). None of the environmental factors studied were associated with acquiring LD, except for people living in a block of flats (vs. individual home) who were at higher risk of developing LD (aOR 2·54, 95% CI 1·75–3·68). Analysis of the factors related to leisure and professional activities revealed that a history of travel with a stay in a hotel (aOR 5·40, 95% CI 3·16–9·22) or in another type of accommodation (aOR 1·89, 95% CI 1·28–2·78) in the 10-day period prior to the onset of the disease were associated with LD. People who exclusively used a wash-hand basin for their personal hygiene were at higher risk of LD when compared to other people included (aOR 1·96, 95% CI 1·21–3·17). These were older (mean age 68·9 vs. 55·3 years, P<0·01) and more often affected by chronic disease or immunosuppression (54·3% vs. 26·5%, P<0·01) than other cases included in the analysis.
Other environment and leisure variables significantly associated with a lower risk of developing LD are shown in Table 1.
Table 1. Univariate analysis of environmental and leisure risk factors for sporadic community-acquired Legionnaires' disease, September 2002 to September 2004, France
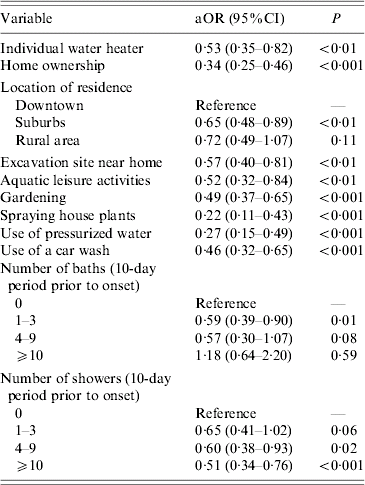
aOR, Adjusted odds ratio; CI, confidence interval.
Multivariate analysis
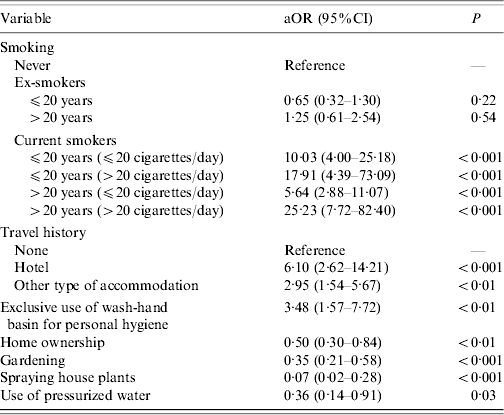
In the multivariate analysis, current tobacco exposure and travelling during the 10-day period prior to the onset of disease remained significantly associated with LD (Table 2). Smoking was a dose-dependent risk factor: cases who smoked >20 cigarettes per day for >20 years were at higher risk (aOR 25·2, 95% CI 7·7–82·4). The odds of illness among travellers increased, with those who stayed in a hotel having a greater risk (aOR 6·10, 95% CI 2·62–14·21) than those who stayed in another type of accommodation (aOR, 2·95, 95% CI 1·54–5·67). The exclusive use of a wash-hand basin for personal hygiene also remained a risk factor in the final model (aOR 3·48, 95% CI 1·57–7·72). Interaction terms were not included in this model, because they were not statistically significant on inclusion in the final model.
Table 2. Multivariate analysis of risk factors for sporadic community-acquired Legionnaires' disease, September 2002 to September 2004, France
DISCUSSION
This study is the first carried out in France investigating factors associated with sporadic community-acquired LD. The risk of developing LD increases with smoking, the numbers of cigarettes smoked per day, and lifetime smoking with a dose-dependent effect. The results also document an association between LD and a history of travel, more particularly with a stay in a hotel during the period of exposure. And finally, the exclusive use of a wash-hand basin for personal hygiene is associated with an increased risk of LD. The multivariate analysis did not show an association between alcohol consumption and LD.
These results confirm the conclusions of several analyses which highlighted the role of tobacco in the development of LD [Reference den Boer, Nijhof and Friesema18–Reference Straus20] and described a dose-dependent effect as previously documented by Storch et al. [Reference Storch19]. However, this dose-dependent effect that combines both number of cigarettes smoked [Reference Storch19] and lifetime smoking had not been documented to date for sporadic community-acquired LD, but only during outbreaks [Reference Greig26] or when considering other respiratory infections [Reference Almirall24, Reference Almirall25, Reference Nuorti27, Reference Gajalakshmi28]. The lifetime smoking seems not to be the most important factor to consider, as the risk is not different between non-smokers and ex-smokers, whatever the duration of their exposure. This dose-dependent effect is in accordance with the known physiopathological mechanisms contributing to an increased sensitivity to respiratory infections among smokers. Tobacco smoking reduces the number of cilia of the laryngeal epithelium and weakens their activity, allowing the persistence of microorganisms in the laryngeal tract [Reference Hirabayashi29]. In parallel, an abnormal mucociliary clearance [Reference Stanley30], and an increased bacterial adherence in smokers compared to that of non-smokers [Reference Piatti, Gazzola and Allegra31] may also contribute to an increased susceptibility to bacterial pneumonia.
One of the first published case-control studies on LD identified alcohol consumption as a risk factor for sporadic community-acquired LD [Reference Storch19]. Excessive alcohol consumption was defined as the consumption of at least three alcohol units per day (regardless of gender). Since that time, and despite heterogenous case definitions, analysis on sporadic community-acquired cases or on cases linked to outbreaks, has not shown any association between the consumption of alcohol and LD [Reference Nguyen10, Reference den Boer, Nijhof and Friesema18, Reference Greig26, Reference Garcia-Fulgueiras32]. However, some exploratory studies showed the potential role of alcohol in community-acquired pneumonia [Reference Ruiz5, Reference Ruiz33, Reference de Roux34] and it would be of particular interest to further study the physiopathological mechanisms which could explain the differences observed and the impact of alcohol on the ability of Legionella to grow, particularly in the macrophages [Reference Yamamoto, Klein and Friedman35].
Another important result of this study is the significant association between a history of travel and LD. This association had already been suggested [Reference Straus20] and documented [Reference den Boer, Nijhof and Friesema18, Reference Storch19] but the type of accommodation was not previously taken into account. Our results highlight that staying at a hotel during the 10-day period prior to the onset of disease is significantly associated with LD. Several studies showed that the proportion of water supplies colonized by Legionella was more important in temporary accommodations than in residential settings [Reference Leoni36–Reference Mouchtouri38]. Similarly, data from the European network (EWGLI) show a high proportion of temporary accommodations (including hotels) for which the investigations report high Legionella contaminations [>1000 colony forming units per litre (c.f.u./l)] [Reference Ricketts and Joseph39].
A recent study analysed the level of contamination of the residential hot water supply of 44 LD patients who had travelled during the 10-day period preceding the symptoms (or for which the residence was not inhabited) [Reference Verhoef40]. These results were compared with those obtained in the residence of 44 LD patients who had not travelled (or for which the residence was occupied). Although non-significant, Legionella were present more often in traveller's homes. This suggests that LD could be due to an exposure when returning from travel. The questionnaire of our study did not allow a precise analysis of the chronology of the travel in relation to the incubation period. In the future, it will be necessary to document these events in order to determine if the risk is related to the travel itself or to the exposure when returning home.
The last identified risk factor for sporadic community-acquired LD is the use of a wash-hand basin for personal hygiene. This result must be interpreted with caution, as data on Legionella contamination in tap water was not available. LD occurs frequently in weakened persons, and the very limited number of cases described relating to tap water were always among the immunosuppressed [Reference Tominaga41, Reference Chen42]. It is probable that this risk applies to a particular subgroup of the population and cannot be generalized. Indeed, cases using the wash-hand basin were older and more often had underlying illness such as chronic disorders and immunosuppression than other cases included.
Being the owner of one's home, gardening, using water under pressure or spraying home plants are associated with a lower risk of LD. As for the variable concerning the use of a wash-hand basin, one can make the assumption that these variables define a particular population that may be less susceptible to LD for other reasons. The meaning of the associations observed needs to be further explored, as there may be surrogate variables for activity or physical fitness. A study conducted in Italy in order to identify potential risk factors for contamination according to distribution systems, showed that old (>10 years) water-heating systems increased the risk for Legionella contamination [Reference Borella43]. Even if not significant, our data analyses show that water-heating systems are more recent among home-owners (11·6 vs. 12·5 years). It will be necessary to perform complementary analyses to document the characteristics of the water-heating systems and the levels of contamination by Legionella.
The present study has some limitations. The sensitivity of the mandatory notification in place in France has improved over recent years but some cases remain unnotified [Reference Nardone22]. Cases included in our analysis were different from those not included for age and outcome (included cases were younger and their CFR was lower). It is possible that cases included were those that were easier to interview, thus presenting a less severe form of the disease. Consequently, our results cannot be completely extrapolated to all the sporadic community-acquired cases of LD occurring in France. Cases and controls were matched according to their underlying condition. Complementary studies would be necessary to better characterize these risk factors. For example, exposure to anti-tumour necrosis factor drugs (e.g. for the treatment of Crohn's disease) was not documented in our study whereas a recent paper highlights the association with LD [Reference Tubach44]. Finally, some confounding variables may have been missed. The exploratory design of the analysis aimed to test a large number of variables and consequently, increased the chance of wrong conclusions, which may explain some results observed (wash-hand basin and surrogate variables for activity or physical fitness).
LD remains an important cause of pneumonia with high mortality. Diagnosis and treatment need to be targeted to patients at increased risk for illness and prevention measures must be focused on risk settings or where there are people at higher risk. The identification of a dose-dependent effect for tobacco is an important finding of this study, as well as the documentation of a greater risk for people with a travel history, particularly with a stay in a hotel. At the same time, research efforts are still needed to better understand the ecology of Legionella and its ability to grow within human-made aquatic environments, the environmental factors influencing viability and spread of Legionella within aerosols, and the relation between clinical presentation and severity of illness.
ACKNOWLEDGEMENTS
This study was supported by a grant from Electricité de France, Service des études médicales (EDF). We thank France Wallet for her support, Lisa King for her valuable review, Christine Cambrezy for assistance in data management, the local health officers who notified and interviewed cases and all the general practitioners who were involved in the inclusion of controls. Institut de Veille Sanitaire, is funded by Ministry of Health, France; European Programme for Intervention Epidemiology Training (EPIET) is funded by the European Commission (DG SANCO).
DECLARATION OF INTEREST
None.




