INTRODUCTION
Fluoroquinolone antibiotics, such as ciprofloxacin, have been recommended by some authorities for the empirical treatment of infectious diarrhoea [Reference Guerrant1]. Such recommendations often carry cautions against overuse, and use when not indicated to minimize risk of the development of resistance in enteric bacteria such as Campylobacter. There are many reports of increasing resistance to fluoroquinolones in Campylobacter strains from humans and foods and food animals [Reference Gaudreau and Gilbert2–Reference Nachamkin, Ung and Li5]. Of specific concern are reports of high levels of resistance in strains in developing parts of the world, and in strains isolated from travellers who have returned from developing countries [Reference Bodhidatta6–Reference Hakanen8]. Case-control studies have investigated risk factors for fluoroquinolone resistance in Campylobacter infections in Europe and the United States. Results vary, but the primary risk factor reported is foreign travel [Reference Engberg7, Reference Kassenborg9, Reference Smith10].
Within southern Alberta are the Chinook Health Region (population 152 000) and the Calgary Health Region (population 1 122 000) [11, 12]. The Chinook region has a large agricultural base and many workers in the livestock industry, while the Calgary region has one of the most rapidly growing metropolitan populations in Canada [13]. The incidence of Campylobacter infection in humans is relatively high in these health regions. In 2000, in both regions there were about 70 infections/100 000; higher than the province-wide incidence of 42·5/100 000 [14]. Thus, these health regions present a good location in which to investigate the relative importance of a number of risk factors for ciprofloxacin resistance in Campylobacter infections.
MATERIALS AND METHODS
Study participants
Study participants were residents of the Chinook Health Region and Calgary Health Region, aged >16 years, who submitted a stool sample that was positive for the presence of Campylobacter, and who were followed-up by a public health nurse or inspector between 1 February 2004 and 29 July 2005. Verbal consent was recorded prior to administration of the study questionnaire. Ethics approval was granted for this study by the Health Research Ethics Board, Panel B, University of Alberta, Edmonton, Alberta, the Conjoint Health Research Ethics Board, University of Calgary, Calgary, and the Chinook Health Region Regional Research Committee, Lethbridge.
Questionnaire description and administration
All questions for the study questionnaire were open-ended and required one-word answers. Potential risk factors included travel within the previous month to destinations outside Canada and the United States, average 2-week consumption of specific foods, frequency of eating in restaurants, drinking unpasteurized milk or untreated water, exposure to animal manure or faeces, administration of an antibiotic to animals or humans, and antibiotics used within the past month. We also included questions about exposure to household products and personal hygiene items containing antibacterial agents, which could increase risk of antibiotic resistance [Reference Levy15]. In addition, we investigated the hypothesized resistance risks associated with possession of antibiotics that the patient had saved for future use (referred to here as possession of non-prescribed antibiotics), which we used as an indicator for the potential for inappropriate self-medication, and living more than 8 km from a pharmacy (rural residence), which could increase the potential for self-medication. Other data gathered included age, sex, education, occupation, details about any antibiotic treatment, and knowledge of antibiotic susceptibility testing results. Public health nurses in the Chinook Health Region and public health inspectors in the Calgary Health Region were instructed on correct administration of the scripted questionnaire. Questionnaires were administered by phone during routine follow-up investigations by the public health officials.
Quinolone susceptibility testing
Susceptibility testing at the Chinook Health Region Laboratory used a modified Kirby–Bauer method, in which colonies were streaked onto Mueller–Hinton agar (BD Diagnostics, Oakville, ON, Canada) with 5% sheep blood (BD Diagnostics) and incubated at 37–42°C for 24–48 h, depending on growth characteristics. The zone of inhibition around a 5 μg ciprofloxacin disk (BD Diagnostics) was assessed as follows: ⩽15 mm, resistant; 16–20 mm, intermediate; ⩾21 mm, susceptible. Campylobacter strains isolated in the Calgary Health Region by Calgary Laboratory Services were tested for nalidixic acid susceptibility using 30 μg nalidixic acid disks (Oxoid, Nepean, ON, Canada) on blood agar plates (PML Microbiologicals, Wilsonville, OR, USA). Susceptibility to nalidixic acid was assessed by zone diameter ⩾20 mm. Plates were incubated at 42°C for 18–24 h.
Data handling
Data were double-entered into SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Cases were participants from whom ciprofloxacin- or nalidixic acid-resistant Campylobacter were isolated, and controls were those from whom ciprofloxacin- or nalidixic acid-susceptible strains were isolated. Illnesses that started at least 2 days after the first day of travel outside the United States and Canada and within 3 days of returning from travel were considered to be caused by foreign strains. This criterion is based on the assumption of a typical 2- to 3-day incubation period for infection [Reference Black16, Reference Blaser17]. When multiple travel destinations were given, the most probable country where an infection was acquired was determined from the individual's travel timeline and illness onset information.
Criteria for empirical treatment with an antibiotic or fluoroquinolone included either a positive response to the question ‘Did you start taking the antibiotic before submitting a stool sample?’ or a course of treatment for the diarrhoeal illness starting ⩾1 days before the date of stool sample submission (extracted from laboratory data). In addition, the patient must have provided the name of their medication, which had to be a recognized antibiotic.
Food consumption frequency data were gathered on a continuous scale, but for modelling purposes, data for most food types were classified as low, medium, or high consumption frequency based on tertiles (three equal divisions of the study population) of the data. Cut-off points for food consumption categorical variables among domestic infections were changed because the distribution of reported frequency of consumption for this group differed from that of the total study sample. Abbreviations for selected variables are given in Table 1.
Table 1. Definition of some study independent variables and abbreviations used in text and tables
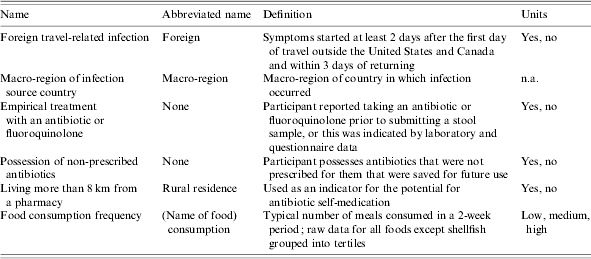
n.a., Not applicable; independent variable has no units.
The study questionnaire is available upon request.
Logistic regression modelling
Univariate logistic regression models were fitted for each independent variable using the SPSS logistic regression function. Along with the crude odds ratios (OR) and 95% confidence intervals (CI) for potential risk factors, ORs adjusted for age, sex, health region, higher education, season, and rural residence were calculated. Three sets of univariate analyses were conducted using the following datasets: (A) all participants, (B) participants with domestically acquired infections, and (C) participants infected from 1 February 2004 to 31 January 2005 (referred to as 2004 infections). Analysis with dataset C controlled any effect of unequal sampling over 2004 and 2005.
A multivariable model was developed using dataset A. Candidate variables were those with a crude (unadjusted) univariate Wald value significant at the 10% level. Final model variables were selected using backwards and forwards stepwise logistic regression model-building approaches, with a P entry value of 0·20, and P removal value of 0·25. Interaction terms were selected from all biologically reasonable pairs of multiplicative effect variables through likelihood ratio testing for a difference at the 10% level and stepwise logistic regression modelling using the previously mentioned entry and removal values. Confounders were identified and added to the base model if, when added to the model, an exposure variable β-coefficient changed by >10%. When the standardized residual for any data-point was outside the 0·1% level of significance, it was removed as an outlier.
Violations of the assumption of sampling adequacy, which is integral to logistic regression modelling, was assessed when >20% of cell values in a contingency table were <5. Multivariable models using datasets B and C were not attempted because >50% of the candidate variables for the base models violated the sampling adequacy assumption. All final results of univariate and model analyses were assessed for significance at the 5% level. Models were assessed with and without inverse probability weighting to adjust for unequal sampling across seasons. Weights were as follows: spring, 0·5, summer, 0·71, autumn, 1·0, winter, 0·65.
Validity assessments
To assess the effect of participation bias, nalidixic acid resistance and travel data for 161 eligible non-participating patients in the Calgary Health Region from September 2004 to July 2005 were acquired from the health region and were compared to participant data. Patient identifiers and any other data from non-participants were not released from the health region. Non-participating patients were those who were aged >16 years and who completed the health region's enteric follow-up investigation but were not asked, or declined to participate in the case-control study. To account for the potential impact of participation bias, the ORforeign was adjusted by dividing with a selection bias factor [equation (1)] [Reference Greenland, Rothman and Greenland18], and this was compared to the unadjusted OR.
where SAF =proportion of participants among cases with foreign strains; SBD=proportion of participants among controls with domestic strains; SAD=proportion of participants among cases with domestic strains; SBF=proportion of participants among controls with foreign strains.
The difference in susceptibility testing methods used in the two health regions was a concern; the less specific nalidixic acid disk method used in the Calgary Health Region could have resulted in an artificially high proportion of ciprofloxacin resistance in Campylobacter strains in that region. Possible resistance misclassification was addressed by applying the Greenland estimation [Reference Greenland, Rothman and Greenland18], [equation (2)], to estimate the number of potentially misclassified ciprofloxacin- resistant strains in the Calgary Health Region. We randomly selected this number of participants from those with resistant strains in this health region, and generated an altered dataset in which susceptibility was imputed for those individuals. This was repeated nine more times and the final multivariable model was run on the ten altered datasets, and the percent difference between the mean OR values from the altered data and the OR values from the original data was calculated. Values for Se and Sp were extracted from the report by Gaudreau & Gilbert [Reference Gaudreau and Gilbert19].
where A=adjusted number of fluoroquinolone-resistant strains; A*=observed number of fluoroquinolone-resistant strains; Fp=false positive probability; N=number of affected strains; Se=probability that resistant strains were classified as resistant; Sp=probability that susceptible strains were classified as susceptible.
RESULTS
Study participants and Campylobacter strains
Based on public health databases from the study area, about 600 cases of Campylobacter infections in people aged >16 years were reported to the health regions during the study period. Manpower shortages in the Calgary Health Region precluded public health inspectors from asking all patients to participate. One Campylobacter outbreak occurred during the study period; outbreak patients were not asked to participate in the study. The number of patients who were asked to participate was 351. In total, 229 patients consented to participate in the study, representing about 38% of all potentially eligible people and 65% of all who were asked to participate. Lack of time was the most common reason given for refusing to participate. Nineteen patients were censored due to lack of stool sample submission data.
The mean age of participants was 40·0 years, 64·8% (n=210) had college or university education, 16·2% reported occupational handling of animals, and 17·1% were rural residents. Among the 210 Campylobacter isolates from study participants, 196 (93·3%) were C. jejuni, six (2·9%) were C. coli, and the species of eight strains (3·8%) was not determined. Most strains (80·5%) were from people reporting in the Calgary Health Region, and 68 infections (32·4%) were acquired outside the United States and Canada, while the remaining 142 were probably acquired domestically. The proportion of ciprofloxacin-resistant strains among all strains was 31·0% (n=210). Resistant strains were more common in infections caused by strains other than C. jejuni, in infections in participants who had higher education, who lived in the Calgary Health Region, and who were male (Table 2).
Table 2. Distribution of ciprofloxacin-resistant Campylobacter cases and ciprofloxacin-susceptible Campylobacter controls in southern Alberta, 1 February 2004–29 July 2005 (n=210)
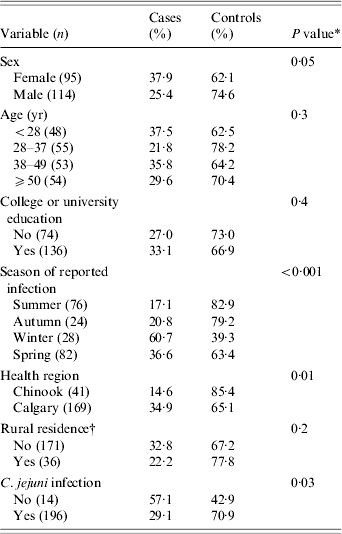
* Significance of the χ2 Pearson statistic.
† Refer to Table 1 for variable description.
Individual risk factors
Results from adjusted univariate models are summarized in Table 3 (all participants) and Table 4 (2004 infections). Two risk factors were associated with ciprofloxacin resistance: recent travel outside Canada and the United States and possession of non-prescribed antibiotics. Furthermore, possession of non-prescribed antibiotics was the only univariate risk factor identified among infections probably acquired domestically (adjusted OR 18·5, 95% CI (OR) 1·8–186·3, P=0·01; n=142). Risk levels were highly variable among travel destination macro-regions (Table 3). Empirical treatment with an antibiotic or a fluoroquinolone, use of antibacterial dishwashing soap or antibacterial toothpaste, contact with animals, and handling of antibiotics were not risk factors for ciprofloxacin resistance at the 5% level. None of the variables for frequency of food consumption were significant risk factors.
Table 3. Univariate analyses of risk factors for ciprofloxacin resistance of Campylobacter strains. Data collected from reported Campylobacter infections in southern Alberta, 1 February 2004–29 July 2005 (n=210)
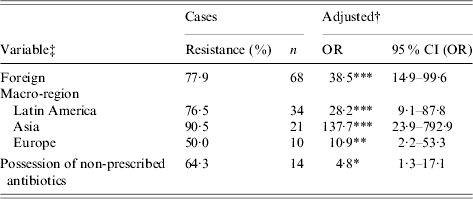
OR, Odds ratio; CI, confidence interval.
† Adjusted for age, sex, higher education, health region, rural residence, and season.
‡ Refer to Table 1 for variable descriptions.
* P<0·05, ** P<0·01, *** P<0·001.
Table 4. Univariate analyses of risk factors for ciprofloxacin resistance of Campylobacter strains from reported Campylobacter infections in southern Alberta, 1 February 2004–31 January 2005 (n=133)
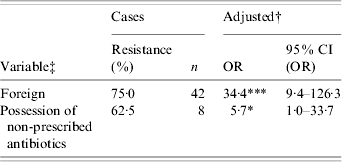
OR, Odds ratio; CI, confidence interval.
† Adjusted for age, sex, higher education, health region, rural residence, and season.
‡ Refer to Table 1 for variable descriptions.
* P<0·05, *** P<0·001.
Multivariable logistic regression analysis
Candidate variables for stepwise model-building included: gender, season, health region, C. jejuni, foreign, empirical treatment with a fluoroquinolone, cattle handling, and possession of a non-prescribed antibiotic. Variables selected by forward and backward approaches for the base model included foreign, possession of non-prescribed antibiotics, and gender. Two interaction terms, gender by possession of non-prescribed antibiotics and foreign by possession of non-prescribed antibiotics, added significantly to the model, but these interaction terms violated the assumption of sampling adequacy and were, therefore, not added to the final model. Potential confounding variables, including higher education, age, season, rural residence, health region, and empirical treatment with a fluoroquinolone, were tested for their effects on base model variables. There was evidence of age confounding the effect of gender, therfore age was added to the base model. None of the other variables listed had a confounding effect.
Four outliers of the final model were identified (standardized residuals for outliers were >3·29 or <−3·29). Final model results, following removal of the outliers, are given in Table 5. With inverse probability weighting for season applied to the data, the changes in coefficients for foreign, possession of non-prescribed antibiotic, and gender were <8%, but changes for two of the oldest age quartiles were >10%.
Table 5. Risk factors for ciprofloxacin resistance identified from multivariable logistic regression model of reported Campylobacter infections (N=205) in southern Alberta, 1 February 2004–29 July 2005
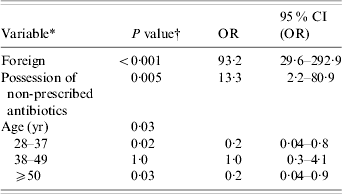
OR, Odds ratio; CI, confidence interval.
* Refer to Table 1 for variable descriptions.
† Significance of the logistic regression Wald statistic.
Study validity
Examination of a sample of eligible Calgary Health Region patients who were not asked to participate or who were asked but chose not to participate showed that the ciprofloxacin-resistance rate was less among non-participants (26·1%, n=161) than among Calgary study participants (33·9%, n=165; χ2=46·5, P<0·001). Among the non-participants in the Calgary Health Region, 19·9% acquired infection in a foreign country, and the crude univariate ORforeign was lower for Calgary non-participants (OR 2·0, 95% CI 0·9–4·5) than for participants in this region (OR 44·3, 95% CI 17·2–114·1). We translated the participation bias effect into a bias-corrected multivariable OR value for foreign travel for the full dataset (we assumed the effect measured was not unique to the Calgary Health Region and would also apply to the Chinook Health Region). The calculated the participation bias factor [equation (1)] as 4·8, so the ORforeign adjusted for participation bias was estimated as 93·2/4·8=19·4.
We evaluated the potential effect of differential misclassification of resistance on the model caused by the use of a surrogate ciprofloxacin susceptibility testing method in the Calgary region. Gaudreau & Gilbert [Reference Gaudreau and Gilbert19] found nalidixic acid diffusion testing had a sensitivity of 100% and specificity of 98·5%, with respect to the gold standard test for ciprofloxacin susceptibility (agar dilution). Based on these values and the Calgary data, the estimated number of correctly classified ciprofloxacin-resistant Campylobacter strains in the Calgary sample was 54 [equation (2)]. This suggested that two of the 56 Calgary infections assumed to be ciprofloxacin resistant may have been misclassified. Ten copies of the original dataset were altered by imputing ciprofloxacin susceptibility for two randomly selected participants with resistant Campylobacter from the Calgary region patients.
Potential misclassification may have biased the original OR estimates both away from and towards the null. With the altered datasets, the average multivariable OR for foreign travel (86·9) was 7% less than it was with the original dataset (93·2) and the average multivariable OR for possession of non-prescribed antibiotics (14·6) was 10% greater with the altered datasets than it was with the original dataset (13·3). Foreign travel was a significant risk factor for resistance with the ten altered datasets, and possession of non-prescribed antibiotics was a significant risk factor with nine of the ten altered datasets. It appears, then, from these simulated datasets in which misclassification was taken into account, the conclusions from the final model were robust against effects of misclassification.
DISCUSSION
The proportion of ciprofloxacin resistance among the Campylobacter strains involved in this study (31·0%) falls between levels reported within the past 5 years in Alberta and Quebec, which range from 2% to 47% [Reference Gaudreau and Gilbert2, Reference Guevremont20, Reference Gibreel21]. Similar to other reports [Reference Guevremont20, Reference Thwaites and Frost22], the levels of resistance in C. coli was higher than in C. jejuni.
This case-control study examined a large number of hypothesized risk factors for ciprofloxacin resistance in Campylobacter infections. The effect of foreign travel has been discussed in many observational studies of antibiotic resistance in Campylobacter infections [Reference Engberg7–Reference Smith10, 23, Reference Gaunt and Piddock24]. Our data showed foreign travel dominated any other risk factors that were examined. Furthermore, stratification of the data by regional group was important because it allowed a more detailed understanding of the risk associated with foreign travel. Cases were almost 140 times more likely than controls to have an infection acquired in Asia, but only 11 times more likely to have an infection acquired in Europe. A larger study with finer geographic groupings (e.g. by country) would probably have allowed us to capture a wide range of risk levels within macro-regions.
It has been shown that Campylobacter are able to develop high levels of resistance to fluoroquinolones soon after exposure [Reference Griggs25, Reference McDermott26], and in a previous case-control study, quinolone use appears to increase risk of quinolone resistance in Campylobacter infections in humans [Reference Smith10]. In this study, 10·8% of cases and 2·8% of controls reported empirical treatment with a fluoroquinolone; however, we could not demonstrate that this was a risk factor for ciprofloxacin resistance.
Self-medicating with antibiotics does occur, and studies report levels of leftover antibiotic possession range between 9% and 38% [Reference Vanden27, Reference Carey and Cryan28]. While antibiotic self-medication is important and often effective in the prevention and treatment of travellers' diarrhoea [Reference DuPont29], and while possession of leftover antibiotics may be due to oversized packaging, i.e. not always due to incomplete courses of previous treatments [Reference McNulty30], the potential for incorrect self-medication with non-prescribed antibiotics to contribute to bacterial resistance is a great concern [Reference Carey and Cryan28, Reference Reeves31]. We agree with McNulty et al. [Reference McNulty30], who suggest that the use of antibiotics may not align with prescribing data, and that this could affect the results of epidemiological studies such as ours.
We ascribed the potential for self-medicating to those who answered ‘yes’ to: ‘Do you have any antibiotics that you would use future illness?’ In our study, possession of non-prescribed antibiotics, which was reported by 6·7% participants (n=210), independently contributed to the likelihood of resistance. Possession was also the only risk factor identified in domestically acquired infections; further demonstrating its distinct effect in the absence of travel. Unfortunately, we did not ask participants who reported possession of non-prescribed antibiotics if they used them. Although 78·6% of those who had non-prescribed antibiotics (n=14) reported that they used an antibiotic for their Campylobacter illness, it is unclear if this was prescribed or non-prescribed use. Furthermore, we could not determine if any non-prescribed antibiotics were taken prior to submitting a stool sample (empirical treatment). Future studies with a larger sample size and higher exposure numbers, as well as more detailed questions on this topic could help to clarify this issue.
In our study, we collected food consumption data in units of typical consumption frequency over a 2-week period, rather than positive/negative consumption prior to onset. Compared to dichotomous consumption data, frequency data allowed for greater discrimination in risk estimates. For example, if chicken consumption was categorized as never/ever the univariate model for resistance would have a β-coefficient standard error of 0·72, while the model using the categorized frequency data had a standard error of 0·18. Nevertheless, unlike others [23], we found that those who consume chicken frequently were not more likely to have Campylobacter infections with ciprofloxacin resistance than were those who consume chicken infrequently. Compared to estimates of quinolone and fluoroquinolone resistance among Campylobacter isolated in retail chicken from other countries [Reference Pezzotti32, Reference Price33], Canadian estimates of resistance are low, ranging from 1% to 12% [Reference Guevremont20, Reference Kos, Keelan and Taylor34, 35]. Potentially, low prevalence of resistance in Campylobacter in chicken may have been a reason behind the lack of risk associated with chicken consumption here. Others have found swimming also contributed to nalidixic acid resistance risk in Campylobacter infections [Reference Engberg7], but we did not find consumption of untreated water, including unintentional consumption while swimming, to be an important risk factor.
We also investigated work-related potential risk factors. Others have found nalidixic acid resistance to be higher in faecal bacteria of livestock workers than in those of non-agricultural workers [Reference Aubry-Damon36]. The proportion of participants in this study who handle animals as part of their occupation was higher than the proportion in the provincial workforce (2·4%) [37], but this type of work was not associated with ciprofloxacin resistance.
The need to examine the role of home cleaning and personal use of antibacterial products in the development of infections from antibiotic-resistant organisms has recently been advanced by several authors [Reference Levy15, Reference Aiello and Larson38, Reference Tan39]. In the present study, the use of antibacterial dish soap and a brand of toothpaste known to contain triclosan, an agent that may be involved in the development of antibiotic cross-resistance, were not found to be associated with resistance. The quality of the data and the validity of these findings may be questionable, since a high proportion of participants, 54% and 29%, respectively, reported using these products. Given the number of dish-soap products and toothpaste brands available, it is seems probable that the frequency of reported use is greater than that of true use. Sensing that the use of antibacterial dish soap is an indication of good hygiene, participants may have been more likely to answer ‘yes’, and the quality of recall of product details among most people is probably modest. A cohort format may be a more suitable than a case-control format to examine the effects of these products on the likelihood of antibiotic resistance.
Other potential shortcomings of this study include the fact that some participants were not blinded to their antibiotic resistance status, which may have introduced a degree of recall bias. Unexpectedly, 37 participants (17·9%, n=207) reported that they knew the results of the antibiotic susceptibility testing conducted on their Campylobacter strain. More than half of those (56·8%) had resistant strains, and this may have affected the way they answered questions regarding exposures to antibacterials and antibiotics.
Participation bias, an increasingly formidable challenge to overcome in observational studies [Reference Hartge40], can involve more than one subset of the population. In our study, there was data missing from non-participants: people who could have, but did not participate and from non-submitters: people who were infected, but did not seek medical help or were not asked to submit a stool sample. We estimated the effect of non-participation on ORforeign and it was considerable. Markedly, among non-participants in the Calgary Health Region, ORforeign was not significant. It is unclear from the data why the effect of travel on resistance was so small among non-participants, but it is possible that, in contrast to participants, non-participants, who were more commonly infected with domestically acquired and ciprofloxacin-susceptible strains, had infections that were more easily treated, and or, shorter in duration and were, therefore, more active at the time of interview and had less time to answer the questionnaire. We therefore suspect that the true effect of travel was less than we have reported. Nevertheless, given the magnitude of the estimated risk, travel was still likely to be a significant factor.
Unfortunately, we had no data to estimate the effect of non-submitters. The lack of data on non-reported intestinal disease, which this study, along with many others, suffers from, leads to results that describe only more severe diseases [Reference Tam, Rodrigues and O'Brien41]. Furthermore, Tam et al. reported that physicians are more likely to ask for stool samples from those who recently travelled abroad [Reference Tam, Rodrigues and O'Brien41]. If this applied to our study population, we can assume that our sample population included, relative to domestic infections, an oversampling of travel-related infections. However, this oversampling would not have biased our risk estimate for travel if the proportion of resistance in travellers and in domestically infected patients in our sample were the same as those in the study population.
Nalidixic acid susceptibility testing is an effective tool for screening fluoroquinolone susceptibility in enteric pathogens [Reference Gaudreau and Gilbert19]. We were satisfied that the Calgary Health Region resistance data was not a source of differential misclassification as the OR estimates for foreign travel and possession of non-prescribed antibiotics were stable in our sensitivity analysis.
In conclusion, results from this case-control study demonstrate the overwhelming influence of foreign travel on the likelihood of fluoroquinolone resistance among Campylobacter infections in southern Alberta, and the possibility that individuals who have personal reserves of antibiotics may, through self-medication, be more likely to be infected with a resistant strain. Increasing frequency of chicken consumption was not a significant risk factor for resistance. A larger study is required to more conclusively test the effects of possession of non-prescribed antibiotics and empirical treatment with a fluoroquinolone, which are uncommon in the population.
ACKNOWLEDGEMENTS
The authors are very grateful for the data collection assistance provided by the staff of Environmental Public Health, Calgary Health Region, including Dr Judy MacDonald, Larry Crowe, Jennifer Jacobsen, Kenning Eng, Sujata Patel, and Sarah Nunn. Thanks are also due to Heather Semeniuk of Calgary Laboratory Services for providing data, and Karen Thomas and the nursing staff of Health Protection, Chinook Health Region for administering questionnaires. J.Y.M.J. was supported by an Alberta Heritage for Medical Research Foundation studentship.
DECLARATION OF INTEREST
None.







