INTRODUCTION
Between 2000 and 2011 over 800 outbreaks were reported to the Health Protection Agency's (HPA) (now Public Health England; PHE) Department of Gastrointestinal and Emerging Infections (GEZI). By far the highest number of outbreaks was caused by Salmonella enterica, which accounted for 391 outbreaks affecting over 10 000 people. Most of these outbreaks occurred in a localized area or single premises, and were therefore investigated by local health protection teams, with support from GEZI if required. Fifty-two confirmed outbreaks (and many unconfirmed outbreaks) crossed regional boundaries during this period, and were defined as ‘national’ outbreaks; these were therefore investigated or co-investigated by GEZI. This article reviews national outbreaks that occurred during the 12-year period and describes how outbreak investigation at GEZI has evolved over this time.
DATA SOURCES
PHE (formerly HPA) is responsible for the national surveillance of communicable diseases in England and Wales. Local clinical microbiology laboratories routinely test diarrhoeal stool specimens from patients for the presence of S. enterica and other gastrointestinal pathogens that fall under the category of ‘reportable diseases’. Presumptive isolates of S. enterica are referred to the Gastrointestinal Bacteria Reference Unit (GBRU) for confirmation and characterization. Data on cases of salmonellosis confirmed by the GBRU populate the national surveillance database. An observed/expected exceedance algorithm is used to detect potential outbreaks using laboratory report data, and these are investigated by the GEZI team. For this review all outbreaks and potential outbreaks occurring at the national level are included.
The PHE's Electronic Foodborne & Non-Foodborne Gastrointestinal Outbreaks Surveillance System (eFOSS) (formerly GSURV, General Outbreak Surveillance System) collects reports of both local and national gastrointestinal outbreaks in England and Wales from local authorities and local health protection teams. These data were used to supplement the national data and to make comparisons with local outbreaks.
NATIONAL vs. LOCAL OUTBREAKS
The focus of this review is national outbreaks, which are defined as outbreaks with cases occurring in two or more administrative regions in England and Wales (with the exception of outbreaks with a clear source in one area, for example a wedding that people from around the country have attended). There are far fewer national outbreaks compared to those occurring in local areas, but they can be very large, in some examples reaching over 1000 reported cases, and reflect a product being distributed nationally. In the review period there were 52 national outbreaks affecting 5934 people, compared to 339 local outbreaks affecting 6972 people, although it is important to note that national outbreaks may incorporate investigations carried out locally and are therefore included in the total numbers for both national and local outbreaks. Where the source of infection was identified, eggs accounted for a high proportion of both national and local outbreaks during 2000–2011 (31% and 30%, respectively); however national outbreaks were more likely to be caused by salad and raw vegetables (38% vs. 3%) and zoonotic transmission (6% vs. 0%) (see Fig. 1).
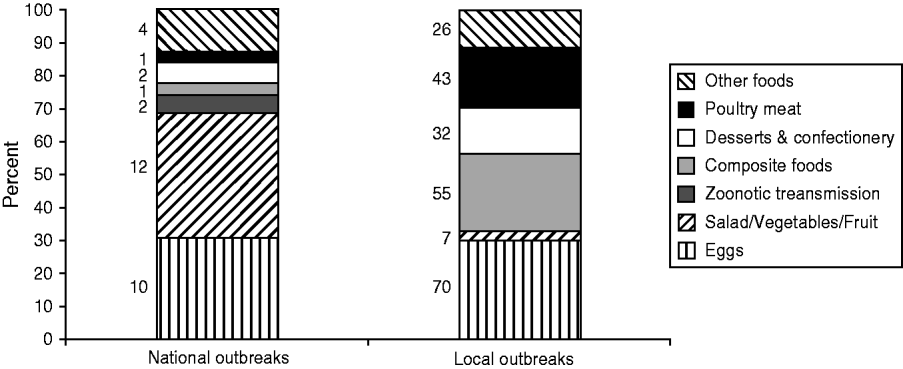
Fig. 1. Proportion of local vs. national Salmonella enterica outbreaks by vehicle of infection (where identified), England and Wales, 2000–2011.
Summary of national outbreaks
Of more than 100 suspected national outbreaks, 52 were investigated at the national level and 32 were linked to a suspected vehicle of infection. This was either because the pathogen was found in food samples, or because analytical epidemiological investigations implicated a food vehicle. Ten outbreaks were linked to eggs and egg products, 12 were linked to salad vegetables and the remaining ten outbreaks were linked to a range of infection vehicles, including two outbreaks linked to zoonotic (animal–human) transmission (see Tables 1 and 2).
Table 1. National Salmonella enterica outbreaks investigated by, or in cooperation with the Health Protection Agency's Department of Gastrointestinal and Emerging Infections, 2000–2011
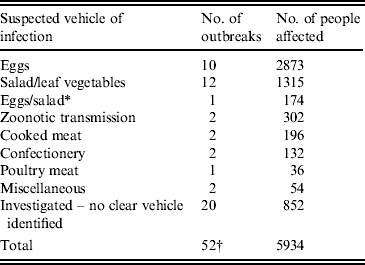
* Egg and salad sandwiches were implicated in the outbreak.
† This total does not include occasions where an excess of a Salmonella subtype is detected, but there is not sufficient evidence to determine whether an outbreak has occurred.
Table 2. Salmonella enterica outbreaks investigated by the Health Protection Agency's Department of Gastrointestinal and Emerging Infections that had a suspected vehicle of infection, 2000–2011
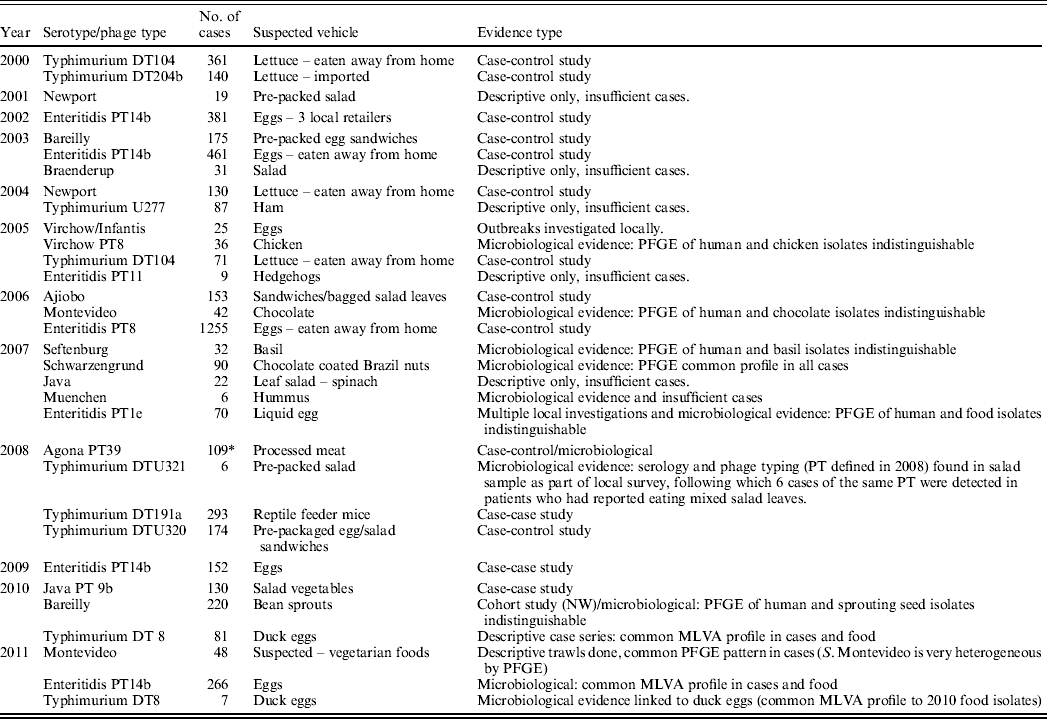
PFGE, Pulsed-field gel electrophoresis; MLVA, multilocus variable-number tandem repeat analysis.
* England and Wales cases only.
Suspected vehicles of Salmonella have been products imported from Europe, the USA, the Middle East, Asia and of course from products originating in the UK. For some products, however, provenance data were unavailable. Of the 32 outbreaks where a vehicle of transmission was suspected, we were unable to trace the contamination to its source for nine, either because a food product came from multiple sources (e.g. in mixed salads or sandwiches) or because a source was suspected but unconfirmed.
Eggs and salad
Eggs continue to be a major source of outbreaks in England and Wales [Reference Janmohamed1–Reference O'Brien4], with the highest number of people being affected by these outbreaks. Major egg outbreaks occurred in 2003, 2006, 2008, 2009, 2010 and 2011 and were also suspected in many local outbreaks. However, as eggs are a common food ingredient it is sometimes hard to trace these back to the source, especially when the eggs are prepared and eaten in the home as opposed to in a restaurant, or contained in a specific salad or sandwich. In 2010 and 2011 two of these outbreaks were linked to duck eggs [both S. Typhimurium definitive phage type (DT)8], reflecting a rise in sales of duck eggs in recent years [Reference Noble5].
Salad vegetables (including fresh herbs and bean sprouts) also continue to be frequently implicated in outbreaks, and have caused the largest number of national outbreaks in the last 12 years [Reference Pezzoli6–Reference Irvine11]. The fact that these outbreaks tend to affect smaller numbers of people than national outbreaks linked to other foods is probably a reflection of their short shelf life and the diversity of products on the market. This highlights ongoing global problems producers experience in trying to keep salad and other ready-to-eat products Salmonella-free [Reference Little and Gillespie12].
There was an outbreak of S. Typhimurium DT U320, in which it was unclear if eggs or salad were the source of infection, as both were contained within the implicated sandwich [Reference Boxall13]. Nonetheless, this highlights the difficulty in implicating specific food vehicles in outbreaks linked to complex or multi-ingredient foods.
Other products
Two outbreaks were investigated where cooked meat was suspected. In one (S. Typhimurium U277 in 2004) a link to ham consumption was suspected, but there were insufficient cases to test this hypothesis in an epidemiological study. The second was an outbreak of S. Agona phage type (PT39) in 2008, headed by colleagues at the Health Protection Surveillance Centre in Ireland. After a relatively small number of cases, they were alerted that the outbreak strain had been identified in an Irish meat processing plant which supplied products to a chain of food outlets across the British Isles. Cases were also identified in England, Wales and Scotland, and a case-control study in Ireland, together with case follow-up in the rest of the UK confirmed the link to this plant. The plant was shut down pending an investigation and was only re-opened when it was certified as Salmonella-free [Reference Nicolay14].
Two outbreaks were linked to confectionery; one to chocolate, and one putatively linked to chocolate-covered Brazil nuts. The first was linked to a major British confectionery company. Forty-five cases of S. Montevideo infection in 2006 [Reference Elson15] were shown to be infected with the same serotype found in products at the processing plant [Reference Elson16], which were subsequently released for sale. A high proportion of the cases questioned (93%) had eaten chocolate from the same company in the days before onset of illness.
The second confectionery outbreak occurred towards the end of 2006, when GEZI was alerted to a cluster of cases with S. Schwarzengrund infection, sharing the same pulsed-field gel electrophoresis (PFGE) profile. Despite hypothesis-generation questionnaires being administered to 18 cases, no hypothesis for infection emerged. However, an identical strain of Salmonella was found in chocolate-coated Brazil nuts the following October. Retrospective review of the hypothesis-generation questionnaires revealed that two cases had reported consuming chocolate-coated Brazil nuts prior to illness. New cases were followed up with questionnaires, but the small numbers of cases reported at that time hampered investigations.
Zoonotic transmission
There have been two outbreaks of Salmonella investigated nationally in the review period linked to animals or animal feed. The first, in 2005 was linked to hedgehogs. Unlikely as it seems, nearly all of the cases questioned reported contact with sick hedgehogs in their gardens or elsewhere, or that the family dog had had contact with one. There was no need to perform an investigation in this case, as the exposure was so unusual, descriptive epidemiology was sufficient.
Another zoonotic outbreak was detected in December 2008, caused by a newly defined type of Salmonella, S. Typhimurium DT 191a. A case-case study showed an association between illness and keeping reptiles, and additional questioning of the cases revealed an association with frozen reptile feeder mice [Reference Harker17]. Salmonella, of a type indistinguishable from that of the cases was found in mice tested from UK supply lines, and these were traced back to a single US supplier. Imports from this supplier were halted, and tighter regulations put in place, as well as updated advice on how to prevent Salmonella infection from reptiles [18].
Investigations with no potential vehicle implicated
Of the 20 outbreaks in which no source was identified, 11 reached the stage where full hypothesis-generation questionnaires were administered to numerous cases but either there were no further cases to trawl, or no clear hypothesis emerged. In the latter situation, trawling is continued into another round of questionnaires and if still no hypothesis is identified then it is difficult to continue the investigation further. This happened for an outbreak of S. Oranienburg in 2009 and for a S. Enteritidis PT12 investigation in 2008, where around 20 trawls were conducted for each outbreak. In most of these instances, there were simply no more cases to trawl, and although a thorough investigation was conducted, the source of the outbreak remained a mystery. This could be because the contaminated food is very common (e.g. chicken) and therefore it is difficult to determine whether cases are eating a disproportionately high quantity of it, if a common ingredient is used in many different foods and therefore difficult to identify, or that the lag time from exposure to interview is too long for patients to recall the contaminated food. The latter is a major problem with organisms where the incubation period is lengthy, e.g. Campylobacter and Listeria monocytogenes and is one reason why fewer outbreaks linked to these bacteria are observed. On other occasions a hypothesis did emerge but cases disappeared before there was time to conduct an analytical study. If there is no food left to test then it becomes difficult to continue the investigation. Due to the time delay between a patient consuming a contaminated food, visiting a medical practitioner, and a specimen being cultured and typed, it is often unlikely that foods will be tested, especially in nationally distributed outbreaks where the vehicle may be widely dispersed.
Unconfirmed outbreaks
The 20 outbreaks with no vehicle of infection identified included in this review do not include the numerous occasions where an excess of a Salmonella subtype is detected, but there is not sufficient evidence to determine whether an outbreak has occurred or not, or where the number of cases returns to the baseline as preliminary investigations begin. National outbreaks of Salmonella in England and Wales are often identified by comparing the number of cases in any given week with the number of expected cases for that time of year, based on the previous 5 years (the ‘exceedence score’). One of the limitations of this method is the dependence on long-term reporting trends, which results in detection of more ‘rare’ serotypes and fewer common serotypes because in those that are very common, detecting outbreaks, especially small ones, becomes more difficult; e.g. S. Enteritidis PT4 commonly associated with consumption of poultry and eggs. Given that eggs are used and consumed by the majority of the UK public, exposure can be viewed as very high. If an infection were to occur in the home, causing a single case, it is less likely to be investigated than five cases eating under-cooked eggs at a cafe. Given the large proportion potentially exposed, the number of these sporadic cases will far outweigh those seen and investigated in specific clusters. Moreover, the exceedance method, which is dependent on a 5-year history of infections, is further limited by the current declining trend in Salmonella infections [Reference Gormley19], meaning that any analysis of current infections may be reviewed against a significantly larger background. This decline reflects more general methods of Salmonella control at the farm level, as eggs and poultry are well documented sources of possible Salmonella contamination.
INVESTIGATION METHODOLOGY
Outbreak investigations affecting more than one region often include more than one distribution outlet, and it is rarely possible to define the entire population at risk. For this reason it is difficult to perform a cohort study, and instead a case-control study is conducted.
Finding suitable controls has become one of the most difficult parts of the investigation. One of the methods used to select controls is ‘random digit dialling’ whereby people who live locally to the case are called and asked to act as controls. This is becoming more difficult as people become more dependent on mobile telephones. Also, anecdotally, it appears to be getting more difficult finding people willing to become involved. This may be due to increased suspicion of cold callers in light of well-publicized scams and the increase in telephone marketing. Random-digit dialling has also proved problematical as the sample can contain a disproportionate number of older people, who are more easily contactable via landline telephones during the day, and this can introduce bias into the study. Furthermore, matching on the telephone exchange may not control adequately for other factors pertinent to the investigation (e.g. socioeconomic status), and if this is not captured during interview then uncontrolled confounding can exist.
There have been attempts to ask cases to nominate a friend or colleague to act as a control. This type of control has been found to be almost impossible to obtain as people are unwilling to give details of potential controls, and attempts to collect controls in this manner have had to have been dropped from at least three investigations in recent years owing to insufficient control numbers.
Difficulties in obtaining controls have lead to different ways of investigation outbreaks, and in some cases the most appropriate solution is to use a case-case study [Reference McCarthy and Giesecke20]. Cases of another infection or even another kind of Salmonella can be used as controls, providing the infection/serotype chosen is not linked to the exposure of interest, and these have proved a very effective method. However, several factors have to be considered with case-case comparisons: ill cases are not being compared with non-ill controls and therefore identified differences in exposure do not represent ‘risk factors’ for illness in the classical sense; it is not possible to make statements about the magnitude or direction of risk in the study population; risk factors for disease which are common to both case groups under comparison will not be identified in these analyses or will be underestimated.
Difficulties obtaining suitable controls are resulting in other methods being investigated by GEZI as alternatives to traditional case-control studies. Using previous trawling questionnaires to provide a ‘bank’ of commonly eaten foods and using randomization of cases’ exposure data as controls [Reference Gillespie21] are all possibilities for future investigations.
Molecular typing
In recent years, the use of multilocus variable-number tandem repeat analysis (MLVA) molecular typing has been used in an increasing number of national outbreak investigations. This method can more clearly distinguish variants of a subtype than traditional methods (stereotyping, phage typing, PFGE), enabling a more accurate case definition, and increased evidence for linking contaminated foods to outbreak cases. MLVA typing has been used for all major outbreaks of serotypes Enteritidis and Typhimurium since 2009, and its success is exemplified in the 2011 S. Enteritidis PT14b outbreak, where the same MLVA profile was found in both the patients and eggs collected from the suspected source of the contamination.
CONCLUSIONS
Over the review period, the annual number of reported Salmonella cases has reduced by almost a third. However, outbreaks still occur at regular intervals and it is essential to continue to actively identify them systematically and investigate thoroughly in order to prevent further cases of infection, and to ascertain which aspects of the production processes are failing. Although the majority of the outbreaks investigated at GEZI are linked with eggs and salad products, salmonellae have been isolated from a wide variety of wild and domesticated animals. These factors, coupled with the fact that Britain sources food globally means that in an outbreak situation, it is essential that investigators keep an open mind and constantly learn and adapt their methods of investigation to ensure they are being conducted as efficiently and effectively as possible.
ACKNOWLEDGEMENTS
The authors thank colleagues from GEZI, GBRU and the Health Protection Units and Regional Epidemiology Units, HPA laboratories and local authorities past and present who helped with these outbreak investigations. Thanks are also due to Dr Iain Gillespie for the information and advice he provided for this review.
DECLARATION OF INTEREST
None.





