INTRODUCTION
Up to August 2007 there were 322 confirmed cases of influenza H5N1 infection in humans, 195 of which were fatal [1]. Human infection with H5N1 virus has been acquired from direct contact with infected poultry and cases have been reported in 12 countries, with more than 10 cases in five of them [1]. Human infection with the H5N1 virus strain was first reported in 1997 in Hong Kong [Reference Claas2, Reference Yuen3]. This outbreak was contained by implementing a mass cull of poultry in Hong Kong; however further outbreaks of H5N1 were identified in poultry in several SE Asian countries in late 2003 and human cases were subsequently identified in Vietnam and Thailand in early 2004 [Reference Chotpitayasunondh4, Reference Tran5]: during 2006, the majority of new cases were reported in Indonesia [Reference Webster and Govorkova6]. The illness experienced has been particularly severe and thus differs from that seen currently in typical winter influenza epidemics: the clinical impact of H5N1 is not confined to the respiratory tract, the virus has been isolated from cerebrospinal fluid, serum, and rectal swabs indicating systemic infection [Reference de Jong7]. The fact that it has occurred in many different countries has heightened concern that this virus might readily evolve into a pandemic strain [Reference Webster and Govorkova6]. Nevertheless the threat of a novel virus giving rise to a pandemic is not confined to this virus [Reference Fouchier8, Reference Peiris9].
Historically the major pandemics of last century have been attributable to viruses with an avian ancestry and have involved three haemagglutinin subtypes: 1918 (H1); 1957 (H2); 1968 (H3) [Reference Potter, Nicholson, Webster and Hay10]. Although we can only speculate about the virulence and transmissibility of a novel influenza virus, contingency planning using the best available information is a necessary response to the pandemic threat. The quality of available information increases substantially with each successive epidemic. In 1918, the influenza virus had not yet been discovered and reliable statistical data on disease incidence and case fatality were not available. By 1968, virology had become a well-established scientific discipline but information on laboratory-confirmed cases remained, and still remains, very limited [Reference Reid11]. The World Health Organisation (WHO) established a network of reference laboratories starting with the World Influenza Centre at the National Institute for Medical Research (London) in 1948, which today is one of four WHO collaborating centres (Tokyo, Japan; Atlanta, USA; Melbourne, Australia) and 118 National Influenza Centres in 89 countries that comprise the global network for influenza surveillance [Reference Kitler, Gavinio and Lavanchy12, 13]. These laboratories have collaborated in monitoring influenza viruses in each annual winter epidemic.
This review is based mainly on clinical surveillance data collected in sentinel general practices in England and Wales over the last 40 years complemented by national statistical data on deaths and hospital episodes [14, 15]. The review aims to highlight experience with particular relevance to influenza pandemic planning, but also to active seasonal influenza management policy.
Royal College of General Practitioners Weekly Returns Service
In 1952 the College of General Practitioners was formed and this stimulated research activities in general practice. At that time, the management of acute infectious diseases formed a major part of the workload in general practice. A small band of research-minded doctors funded the Epidemic Observation Unit and held meetings sharing their interests in the spread of infectious diseases. At the same time the College established the Records and Statistical Unit which was the embryonic Birmingham Research Unit. Ledger-based age/sex and disease registers opened the door to systematic monitoring of consultations in primary care and facilitated disease surveillance in a systematic and consistent manner.
The Weekly Returns Service (WRS), operating from the Birmingham Research Unit, is now based on a network of over 100 practices (about 500 doctors), monitors a population of 955 000 (200 000 in 1967) and delivers a twice-weekly contemporaneous report on the incidence of new episodes of illness seen by enrolled general practitioners (GPs) [16]. The individual practice-specific datasets are stored on a database and provide the opportunity to investigate the experience of 1969 and subsequent years in relation to influenza and other respiratory illnesses. Doctors contributing to the WRS are required to register the clinical diagnosis(es) at each consultation together with the episode type distinguishing new episodes from follow-up consultations. Diagnoses are clinically based and although guidance is given on the choice of terms, precise definitions are not set. The procedure is now based on routine electronic patient records with diagnoses registered as Read codes. The data are transferred electronically as tabular summaries to the Birmingham Unit for analysis.
LESSONS
The lessons deduced from this review of the last 40 years influenza-related experience are summarized under appropriate headings and discussed as each is presented.
Incidence of influenza-like illness
The term influenza-like illness (ILI) refers collectively to a group of clinically diagnosed symptoms commonly associated with influenza virus infection and has proved a reliable indicator of virus-confirmed influenza in the same population [Reference Fleming17, Reference Zambon18]. Persons with ILI present with a wide variety of symptoms depending on age, the duration of symptoms at presentation, the virus type/subtype and the effects of comorbidities. The incidence of ILI since 1967 is presented as the mean weekly rate in each 4-week period; thus there are 13 bars in each annual dataset (Fig. 1; also included within this figure are the influenza viruses responsible for a selection of winters with significant epidemics of ILI activity). The pandemic of 1968/69 (Hong Kong influenza due to an influenza A H3N2 virus) had maximum impact in the United Kingdom in midwinter 1969/70. In the first week of 1970 the incidence of ILI was reported in the WRS at 1252/100 000, a rate which has not been equalled since. Throughout the 1970s there were substantial influenza epidemics in most winters but subsequent epidemics, although seen in most winters, have been much less severe [Reference Elliot and Fleming19]. Doctors entering general practice after 1990 have not experienced a serious influenza epidemic.
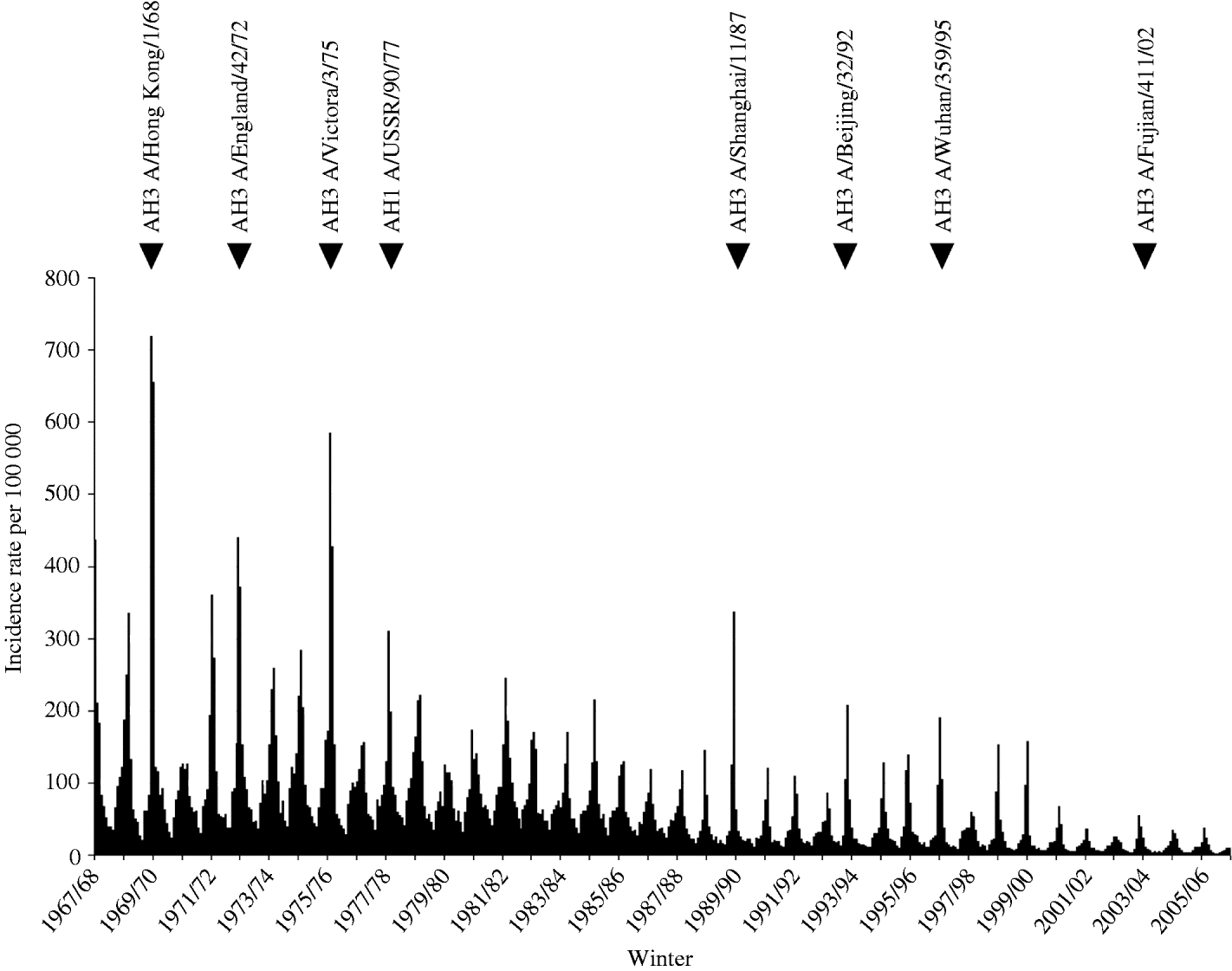
Fig. 1. Influenza-like illness (ILI) 1967/68 to 2006/07: mean weekly incidence rate per 100 000 population in 4-weekly periods. The influenza viruses (type, subtype and strain) are highlighted for winters of significant activity.
In the decade following the 1969/70 UK pandemic, there were several winters in which high levels of incidence were reported. The introduction of the next pandemic virus is also likely to be followed by several years of increased influenza activity and increased demands for vaccination and prophylactic treatment. The gradual decline in the magnitude of epidemics is not explained, but could perhaps reflect a diminishing capacity of the present H3 viruses to drift to any further substantial degree. The major part of this decline occurred well before the policy of routine influenza vaccination of the elderly was established.
Current influenza vaccination and management strategies in the United Kingdom are determined by the Department of Health following advice provided respectively by the Joint Committee on Vaccination and Immunisation and by the National Institute for Clinical Excellence [20]. Strategies are based mainly on the cost effectiveness of interventions but the evidence base is derived mainly from experience of influenza in the 1990s. Management strategies not only for an initial pandemic year but for the subsequent years need to take account of the difference between recent epidemics and those of 30 years ago. In particular, the pressure on hospital admissions (see below) and the cost consequences from increased numbers of persons with severe illness will radically change the cost benefit of extended vaccination and recommended treatment policies. There is also a need to make influenza management policies more consistent. Currently, patients in good health with influenza are often advised to stay at home, drink plenty of fluid and take paracetamol. For the elderly and those with risk morbidities, advice includes encouraging vaccination at the appropriate time and antiviral treatment if they get influenza. Whilst it will always be the case that persons with existing illness are at greater risk, the notion that influenza is of minor consequence needs to be dispelled. Minor illnesses experienced by large numbers of people are a major health management problem even if the case-fatality rate is low.
The duration and spread of epidemics
During the 20 years (1967–1986) there were six epidemics in which the weekly incidence rate of ILI peaked above 400/100 000. In the last 20 years (1987–2006) this rate was exceeded only once (1989/90) and altogether there have only been 6 years in which the maximum weekly incidence rate exceeded 200. Influenza activity is seen every winter but its timing varies considerably: epidemics do not occur in summer although there may be isolated outbreaks. In the last 20 years some of the more serious epidemics have peaked well before Christmas (1989/90, 1993/94, 1995/96), although in the 1970s some severe epidemics occurred much later (1969/70, 1975/76, 1976/77, 1978/79). Current vaccination policy encourages doctors with payments, provided that target levels of vaccine uptake are achieved. Thought should be given to attaching a time limit to target achievement. There is an approximate 2-week gap between vaccine administration and effective immunity [Reference Lambkin21]: accordingly, a desirable vaccination policy should be directed at vaccination by mid-November at the latest.
The rise and fall of incidence during influenza epidemics has been examined in data derived from surveillance in the WRS, in a similar Dutch sentinel network [Reference Fleming17] and in some other countries reporting to the European Influenza Surveillance Scheme [22]. Epidemics rarely last more than 10 weeks in any one country and do not occur in summer months whether North or South of the Equator. The pattern of spread in three regions of England and Wales (North, Central, South) has also been studied using data from the WRS. There is very little regional difference between the onset and peak of the more severe epidemics. Pandemic planning therefore cannot allow for management teams moving from one part of the country to support another.
Reported incidence of ILI in the WRS in February of winter 1968/69 was probably the first indication of the Hong Kong pandemic strain in this country and yet incidence petered out as summer approached re-emerging with much greater impact in the following winter 1969/70. This pandemic virus was first reported from Hong Kong in July 1968 and hit most countries in the winter of 1968/69 [Reference Potter, Nicholson, Webster and Hay10]. There is some controversy as to the origin of the 1918/19 H1N1 pandemic but in this pandemic also, an initial mild wave in the spring of 1918 was followed by two waves in the autumn/winter of 1918 and spring 1919 [Reference Reid11].
The importance of age
The weekly incidence of ILI is described by age for a selection of the more severe epidemics over the last 40 years (Fig. 2). For each of the selected seasonal epidemics we identified the peak week of incidence in all ages combined (week 0) and here present data for 11 weeks including the five previous and five succeeding weeks. Age-specific incidence rates of ILI per 100 000 population are given. In 1969/70, the main pandemic winter, incidence was maximal in persons aged 45–64 and 15–44 years (the working population); in 1989/90 and 1993/94 in children; and in 1999/00 in the elderly. In the pandemic of 1918/19, particularly high mortality rates and severe influenza also hit persons of working age. In the millennium winter (1999/00) ILI was almost confined to adults, rates in school children scarcely rising above the rates in non-influenza winter periods. Rates in pre-school children were also low. These observations seriously challenge the widely held view that children drive the spread of influenza in the community. Differences in the incidence rates according to age are also seen when winter seasons are analysed by the dominant virus type/subtype. In influenza B epidemics, rates in school children are usually the highest; in influenza A H1N1 epidemics rates are highest in pre-school children, but in the H3N2 epidemics there is no clear consistent pattern [Reference Elliot23].
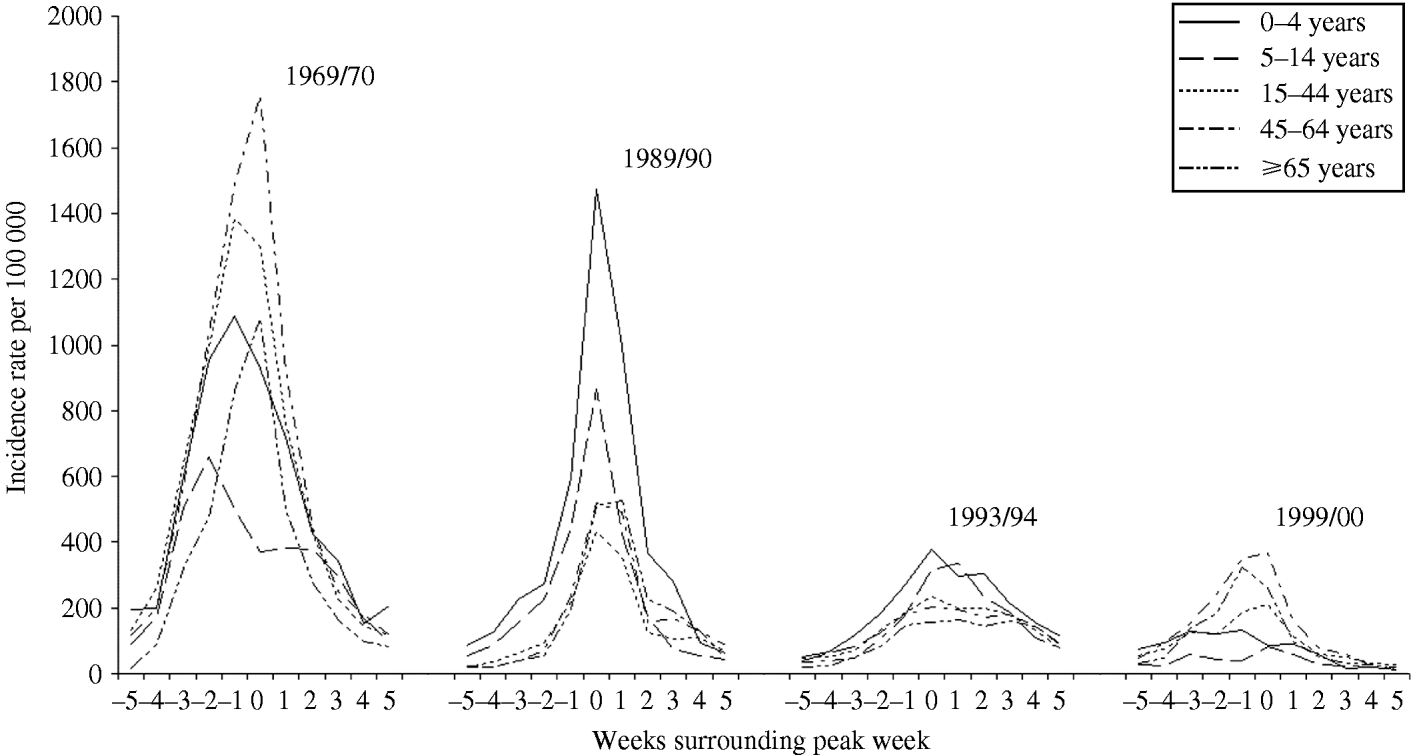
Fig. 2. The weekly incidence of Influenza-like illness (ILI) described by age for a selection of the more severe epidemics over the last 40 years. Age-specific rates are presented for the weeks surrounding the peak week of all-age incidence (week 0).
Until we have information on the impact of any new virus, it is impossible to establish which persons should be prioritized for treatment and vaccination. Furthermore, in formulating management strategies we must not ignore the likelihood of a seriously depleted workforce to implement policy. Plans must include the extension of selected traditional medical and nursing tasks to other personnel.
Health-care delivery
In 1969, virtually all community-based care was delivered by GPs and there was little opportunity for direct admission to hospital with an acute respiratory infection other than by referral. Twenty-five percent of all consultations were provided as home visits whereas today it is probably nearer 2·5%. The tradition of regular visits to the chronic sick was strong in most practices but in most this has now ceased. The elderly and young children were most likely to be visited and a pyrexial illness was a common reason for requesting a home visit.
Over the last 15 years there have been considerable changes in the delivery of care. In the 1990s GPs became increasingly involved in rota systems for emergency work, first sharing with their practice partners, later by involvement in cooperatives in which they played an active part, and then in subcontracting their emergency responsibilities to independent deputizing services. The recent contract revision for GPs has resulted in many practices giving up any role in providing out-of-hours care. Persons requesting services outside normal office hours are directed through a phone call filter into arrangements for direct hospital attendance, review in primary-care centres (often based in hospital emergency departments), or a home visit by a doctor who has no follow-up responsibilities. At the same time there have been changes in the population structure with increased numbers of elderly persons, increased longevity, many more persons living alone, greater dispersion of families with reduced potential to support sick and aged relatives, and more persons in sheltered and welfare supported care in circumstances falling short of nursing home requirements.
The net effect of these factors has been to increase the pressure on admission to hospital in the event of an acute respiratory infection, a phenomenon illustrated in Fig. 3 in persons aged ⩾65 years, using WRS data on new episodes of respiratory illness, Hospital Episode Statistics (HES) data on respiratory admissions and Office for National Statistics (ONS) data on all-cause deaths. Regression analysis shows a sharply decreasing trend of episodes reported in general practice a modest downward trend of death rates and an increasing trend of hospital admissions. This illustration highlights the danger of misinterpretation of trends when sources of data are considered in isolation from other relevant material.
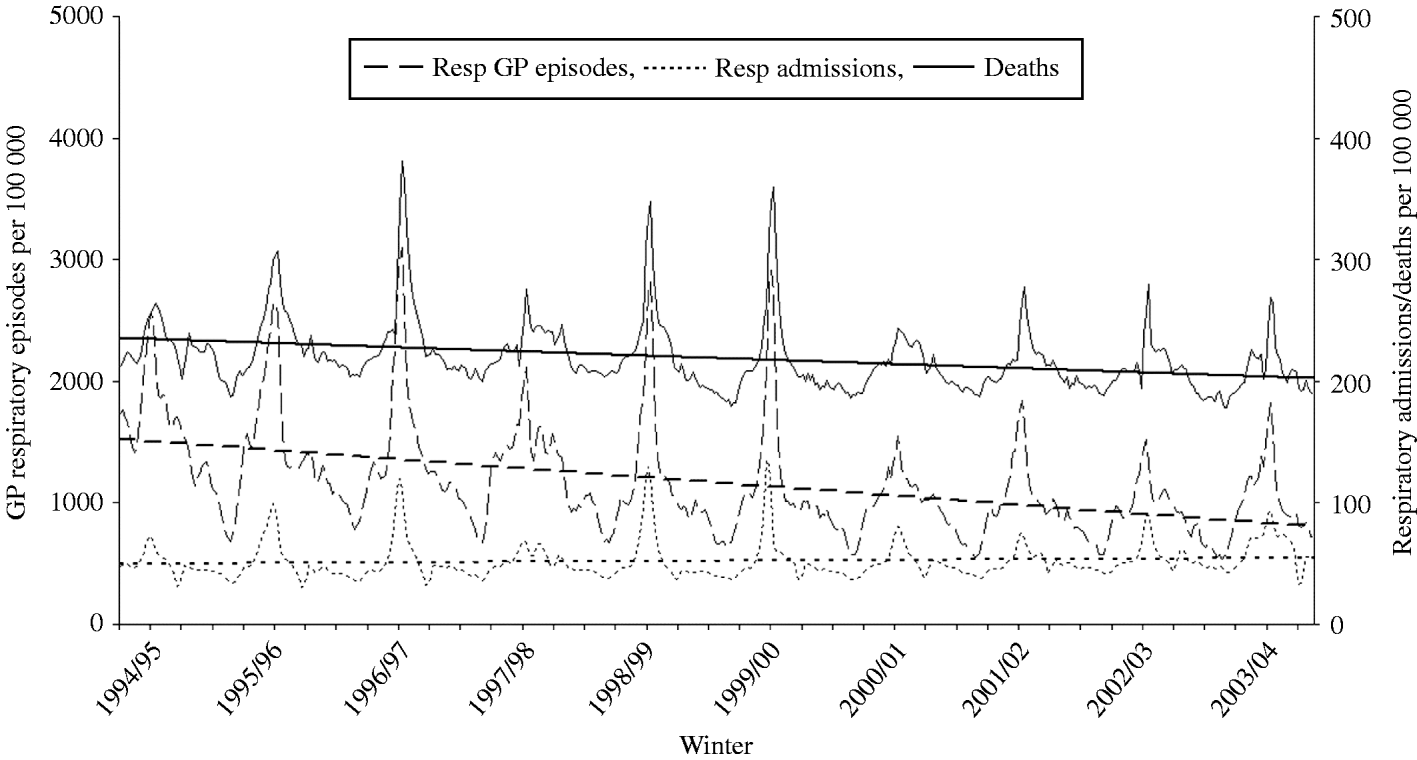
Fig. 3. Respiratory general practitioner (GP) episodes and respiratory hospital admissions in the elderly (⩾65 years), and all-age all-cause deaths from 1994/95 to 2003/04. Regression slopes have been superimposed on each series to highlight the conflicting trends.
The importance of age in relation to hospital admissions is shown in Fig. 4. HES data for England were used to estimate excess respiratory admissions during periods of influenza activity over the years 1989–2001 and the results of that analysis are presented in 5-year age bands [Reference Fleming, Harcourt and Smith24]. The average bed occupancy is calculated from a sample of admissions and applied to the national estimates of excess admissions. The main points for planning purposes are that more than half the admissions and two thirds of the bed days were taken by persons aged >70 years. These data do not relate to a pandemic year but unless these people are managed effectively, our hospital services will not stand the strain of a pandemic nor of the epidemics that are likely to follow. If admission pressures are to be contained in a pandemic situation, it is likely that we will need to return to the style of primary care delivered several years ago. At the least, the essential focus will need to be placed on seeing patients in their own homes (and not already halfway into hospital), an effective continuous service with cover 24 h a day, and securing appropriate follow-up using whatever staff are available.

Fig. 4. Average excess admissions (![]() ) and excess bed days (–––) in influenza epidemic periods by age group over years 1989–2001.
) and excess bed days (–––) in influenza epidemic periods by age group over years 1989–2001.
Case presentation
The interval between symptom onset and case presentation is critical if neuraminidase inhibitor (NI) antiviral therapy is to be used effectively. Clinical trials of treatment using both zanamivir and oseltamivir have shown benefits only where these drugs have been given within 48 h of symptom onset. Whilst it is premature to speculate on the appropriate use of these drugs in a pandemic, early administration will be important [Reference Aoki25].
In a study of 918 persons presenting to GPs and diagnosed as having ILI, only 23% consulted within 48 h of symptom onset and these were mostly children [Reference Ross26]. Only 13% of persons aged ⩾65 years were seen within 48 h of onset. The interval between consultation request and appointment was such that 82% of persons were seen within 24 h of request. Thus the main cause of the delay rested with the patient and not the health-care system. The delay between symptom onset and presentation covers the time period in which viral shedding is highest and spread is most likely [Reference Gentile27]. Even in patients attending a pharmacy only a third of patients (69/217) attended within 48 h [Reference Fleming28]. Patients have been discouraged from presenting with acute respiratory infections. Effective pandemic management will require radical changes of attitude on the part of patient, doctor and health-service provider and these cannot be achieved overnight. Part of the answer lies in changing the perception of influenza as a minor self-limiting illness, but part also lies in making much wider use of antivirals now. Although isolation is not a realistic policy for such a common illness, persons with febrile illnesses during influenza epidemics should not stay at work and should minimize social contacts.
Deaths
In the pandemic year 1918, the all-age influenza death rate rose to an unprecedented level of 3129 per million population and in 1919, 1170 per million which compares with equivalent rates of 250–500 during the previous 25 years [29]. The previous pandemic to that of 1918 occurred in 1890 and there were some sharp differences in the age-specific composition of fatal cases. In 1890, deaths in children (0–15 years) accounted for about 10% of all deaths compared with 25% in 1918/19; in persons 15–35 years 10% compared with 45%; and in persons aged >55 years 65% compared with 10%. Today, when assessing the impact of influenza we assign less significance to the certified cause of death and study either all-cause or all respiratory mortality recognizing that the impact of influenza is seen across a wide spectrum of diagnoses. Mortality attributable to influenza is much higher in a pandemic and the years immediately following one and in particular, higher in children and young people [Reference Simonsen30]. In the typical winter epidemics of recent years influenza-attributable deaths in England and Wales (all causes, all age groups) have been estimated to average 12 000 annually [Reference Fleming31], a rate-based estimate comparable to that in a recent major American study [Reference Thompson32]. Some deaths in children occur in every epidemic but are greater when a novel virus or major drifted variant is seen [Reference Fleming, Pannell and Cross33, Reference Bhat34].
The confounding effect of other viral pathogens
Structured sentinel surveillance combined with integrated virological testing can provide the underlying aetiological causes of the clinical respiratory diagnoses made in the community [Reference Zambon18]. In the epidemic and pandemic situation, laboratory testing can provide early strain characterization of dominant strains and information on the antiviral susceptibility of those viruses. Limitations of testing include the characterization of a small fraction of the viruses circulating, possible selection bias for more susceptible patients, e.g. young infants, and reduced positive results due to delays in patient presentation and transport of samples to the laboratory.
Influenza is just one of the respiratory pathogens seen in most winters. Among others respiratory syncytial virus (RSV) is particularly important [Reference Fleming, Elliot and Cross35, Reference Fleming36]. RSV appears much more consistently than influenza and in the UK epidemics usually begin in December [Reference Goddard37]. However, our understanding of the impact of RSV in adults is very limited because 95% of virological specimens for the investigation of RSV come from children. The incidence of acute bronchitis in children as reported to GPs in the WRS is closely and contemporarily associated with RSV reports received by the Health Protection Agency. The incidence of acute bronchitis in persons aged ⩾65 years follows that in children by 2–3 weeks which differs from the situation with ILI where there is no similar lag [Reference Fleming38].
In some winters, reports of ILI and acute bronchitis in persons aged ⩾65 years rise to a peak together (1999/00); in some there is a clear separation (bronchitis before ILI in 2002/03 and ILI before bronchitis in 2003/04). Weekly data on hospital episodes of respiratory disease, deaths from respiratory disease and episodes of ILI and of acute bronchitis reported in the WRS in persons aged ⩾65 years are shown for these example years in Fig. 5. These are displayed against a background of influenza and RSV active periods defined from those weeks that embrace 70% of all positive virus reports to the Health Protection Agency in the respective winters (and in the case of RSV, lagged by 3 weeks reflecting the delay in the incidence of acute bronchitis between children aged 0–4 years and the elderly aged ⩾65 years [Reference Fleming, Elliot and Cross35]. When the incidence of these illnesses coincides, the pressures on health-care services compound each other. This phenomenon was particularly evident in the millennium winter (1999/00), a year in which our health services were severely overstrained. The incidence of acute bronchitis in the elderly reached the maximum level reported in the history of the WRS whereas the all-age incidence of ILI was not exceptional. The evidence suggesting that acute bronchitis is caused by RSV is substantial in children and is accumulating in the elderly [Reference Thompson32, Reference Fleming, Elliot and Cross35, Reference Falsey39]. The reduced severity of influenza in recent years and the impact of improved vaccination have exposed the extent to which winter hospital pressures and excess mortality may be attributable to viruses other than influenza [Reference Jefferson40]. In the context of pandemic planning, the simultaneous circulation of other pathogens will also take place. The probability of RSV activity around the turn of the year needs to be factored into plans for pandemic management should the pandemic virus appear at that time.
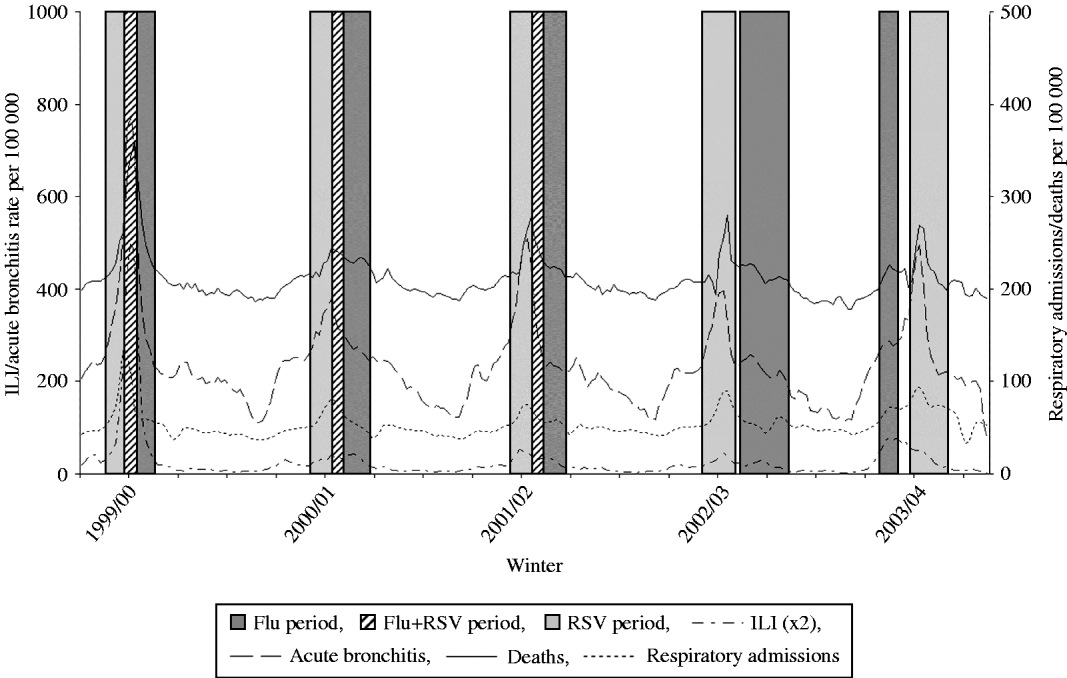
Fig. 5. Clinical incidence of influenza-like illness (ILI) and acute bronchitis (⩾65 years), respiratory admissions (⩾65 years) and all-cause deaths (all-age) with influenza and respiratory syncytial virus (RSV) active periods superimposed for the years 1999/00 to 2003/04.
CONCLUSIONS
There is good evidence to believe that an influenza pandemic at some time is almost certain but when that will come, which virus strain will be involved and how severe the clinical manifestations will be are speculative. The recent emergence of infection in humans acquired from poultry and caused by an avian H5N1 virus has raised the spectre of an imminent pandemic. The clinical surveillance of influenza particularly from the perspective of health-care utilization over the last 40 years in England and Wales holds a number of lessons for pandemic management, some of which are summarized here.
• There is considerable variation in the impact of influenza in different age groups in each epidemic. Preparations for a pandemic must include the possibility that the maximum impact will be felt in the working population.
• The evidence base for estimating the cost effectiveness of vaccination and treatment of influenza is seriously limited being mainly established during recent years of relatively minor epidemics. The less robust data available from the 1970s are more relevant to policy decisions.
• Severe epidemics of influenza have often started well before Christmas emphasizing the need for routine winter vaccine administration by early November.
• Only a minority of patients with influenza present in time to benefit from treatment with neuraminidase inhibitor antiviral drugs. Effective management of influenza involves changing the perception that flu is a minor self-limiting illness which does not require treatment.
• Hospital admissions because of acute respiratory infection occurring in elderly persons have increased in recent years whereas incidence in elderly persons presenting to GPs and deaths due to respiratory disease have decreased. Changes in the delivery of health care have increased rather than decreased the likelihood of admission. Radical changes in primary care restoring traditionally accepted aspects of general practice: for example, ready availability and response to see (and treat) acute respiratory infections early in the course of infection, doctors seeing acutely ill patients within their own homes (day or night), and ensuring seamless follow-up of ill patients.
• Influenza epidemics impact in all regions of the country within a similar time-frame and usually last about 10 weeks. In the event of a pandemic, there will be little opportunity to redeploy manpower resources to other regions.
• The compounding pressure from other respiratory viral infections, notably RSV, needs to be considered if a pandemic strikes in mid-winter.
ACKNOWLEDGEMENTS
We gratefully acknowledge the contribution of the WRS sentinel practices and their staff in providing the general practitioner episode data. The Birmingham Research Unit of the Royal College of General Practitioners is funded by the Department of Health. A.J.E. is jointly funded by the Royal College of General Practitioners and the Health Protection Agency.
DECLARATION OF INTEREST
D.M.F. has served on advisory boards to vaccine and antiviral drug manufacturers and received lecture fees and financial support to attend conferences related to influenza prevention and treatment.







