INTRODUCTION
Norovirus (NoV) is a common cause of acute gastroenteritis. The virus is highly contagious and is transmitted from person to person or via contaminated food or water [Reference Glass, Parashar and Estes1]. Transmission of pathogens via municipal drinking water may affect a large population and thereby cause considerable economic and health impact [Reference Baker2–Reference Werber4]. During the last few years NoV has been identified in an increasing number of waterborne outbreaks in Sweden [Reference Nygard5, Reference Riera-Montes6] and waterborne outbreaks with NoV have also been reported from many other countries [Reference Werber4, Reference Hewitt7–Reference ter Waarbeek9]. This increase in reported NoV waterborne outbreaks may reflect the improved molecular diagnostic methods now available for detection of the non-cultivable NoV in environmental waters, and in patient samples. NoV are genetically classified into five genogroups, known as GI–GV, where strains of NoV GI, GII and GIV can cause infections in humans [Reference Atmar10]. Within a genogroup, NoV strains are further divided into a number of genotypes on the basis of capsid nucleotide sequence [Reference Zheng11]. Although NoV GI strains tend to dominate in water-related outbreaks both NoV GI and GII and mixtures of NoV strains have been reported from waterborne infections [Reference Werber4, Reference Nygard5, Reference ter Waarbeek9, Reference Maunula, Miettinen and von Bonsdorff12] whereas GII strains predominate in reports from institutional outbreaks [Reference Kremer13, Reference Matthews14].
NoV are excreted in the faeces of infected humans and therefore found in sewage and contaminated surface water, which in turn may occasionally contaminate drinking water systems resulting in waterborne outbreaks [Reference Astrom15, Reference Maunula16]. Discharges of treated and untreated municipal wastewater and heavy rain are important risk factors for peak concentrations of pathogens in surface water [Reference Kistemann17]. In contrast to the indicator bacteria, such as Escherichia coli, which are used for measuring the quality of drinking water, the non-cultivable human NoV are described as moderately resistant to chlorination and may therefore be present in spite of the presence of chlorine [Reference Keswick18, 19].
The impact on the whole community may be significant during a waterborne outbreak. In particular the economic losses associated with the outbreak may be substantial depending on the characteristics of the outbreak including the causative agent, the number of people affected and the duration of the outbreak [Reference Corso3]. Early identification of waterborne outbreaks and prompt establishment of control measures may play a major role in reducing the total number of sick individuals and the economic impact of the outbreak.
A large waterborne outbreak of NoV occurred in Lilla Edet in Sweden in 2008. The molecular characteristics of the NoV strains from patients in this outbreak have been reported previously [Reference Nenonen20]. In this study we describe the outbreak from an epidemiological perspective, also taking the costs associated with the outbreak into consideration.
METHODS
Outbreak description
On 11 September 2008, the County Medical Officer (CMO) was informed about an unusually high number of individuals who had suddenly fallen ill with gastrointestinal symptoms during the previous days in Lilla Edet, a small municipality in southwest Sweden. Cases of gastroenteritis were reported from different places in the municipality, e.g. from the nursery schools, schools, nursing homes and from the Primary Healthcare Centre (PHC). The CMO notified the Environmental Office in the municipality about the ongoing outbreak. As the initial information indicated that drinking water was a possible source of the outbreak a boil water recommendation was issued on the same day.
Of the 13 000 inhabitants in Lilla Edet about 7500 are supplied by drinking water from the Lilla Edet water treatment plant (WTP). Other households in Lilla Edet are supplied by another WTP or by private wells. It became obvious by the second day of the outbreak that cases with gastrointestinal symptoms were concentrated in households supplied by Lilla Edet WTP and the boil water recommendation was amended to include only households supplied by this WTP.
The WTP in Lilla Edet uses raw water from the Göta Älv river with microbial barriers in the treatment process including pre-chlorination, coagulation/direct filtration and post-chlorination. August 2008 was a month with unusually high precipitation in the Göta Älv river valley and during the first week in September heavy rains continued to fall on the already saturated ground. As a consequence of heavy rainfall events at the end of this extremely wet period, eight combined sewer overflows were activated in the municipality of Trollhättan, located 20 km upstream, and six combined sewer overflows in Lilla Edet were activated upstream of the water intake to the Lilla Edet WTP. Moreover, on 2 September, untreated wastewater was released into one of the tributaries upstream of the intake due to an emergency discharge [Reference Heinicke21].
An outbreak investigation team was promptly formed, initiated by the CMO, and included representatives from Lilla Edet municipality, National Food Agency, County Administrative Board, National Water Emergency Team (VAKA), Swedish Institute for Communicable Disease Control (SMI), Department of Clinical Virology at Sahlgrenska University Hospital, Lilla Edet PHC, Department of Communication, and Department of Communicable Disease Control and Prevention, Region Västra Götaland.
Investigations were initiated to identify the causative agents, the extent of the outbreak and possible sources of infection. Efforts were also made to inform the inhabitants in the municipality about the ongoing outbreak, the issued boil water recommendation, and medical advice for people with gastrointestinal symptoms. Information was spread via the media, the homepage of the municipality website, posters and local radio as ‘VMA’, i.e ‘important message to the public’.
In addition to the cases reported from the municipality of Lilla Edet, the CMO was notified that 7/17 athletes of a team that had visited the municipality during the afternoon on 7 September had fallen ill with gastrointestinal symptoms 2 days after their visit. The athletes were reported to have drunk municipal water and not eaten during their short visit.
Epidemiological investigation
To estimate the proportion of the 13 000 inhabitants in Lilla Edet that fell sick during the outbreak, questionnaires were sent by mail on 19 September to 1199 randomly selected inhabitants between the ages of 19 and 75 years. A follow-up reminder letter about the questionnaire was sent out about 2 weeks later. The inhabitants aged ⩽75 years represented 93% of the population. The municipality of Lilla Edet includes seven minor geographical areas with separate postal codes, which were all included in the sampling. The questionnaire included questions about all members, i.e. both children and adults, in the household. Questions were asked about how many individuals in the household had had acute gastroenteritis during 5–21 September, date of onset of symptoms, if the household was supplied with municipal drinking water or private well water, and how many glasses of drinking water on average each member in the household consumed per day.
A case was defined as a household member with acute gastroenteritis with date of onset between 5 and 21 September. A control was defined as a household member without acute gastroenteritis during the same period. All inhabitants were divided into two groups, those who lived in households supplied with drinking water from Lilla Edet WTP, and those supplied with drinking water from other sources, i.e. from another WTP, or from a private well.
Odds ratios (ORs) and 95% confidence interval (CIs) were calculated for the risk of being a case and having been exposed to drinking water from Lilla Edet WTP and being a case and not having been exposed. ORs and CIs were also calculated for the average number of glasses of water from Lilla Edet WTP consumed per day and being a case. All analyses were performed using R version 2.7.1 (R Foundation, Austria) and a P value <0·05 was considered statistically significant.
Microbiological investigation
Fifty stool samples from outpatients with symptoms of acute gastroenteritis attending Lilla Edet PHC during 12–26 September were collected and examined for enteric pathogens, as described by Nenonen et al. [Reference Nenonen20].
Water samples, i.e. raw water from the Göta Älv river, drinking water from different parts of the distribution network, and water from reservoirs in Lilla Edet, were collected on 12 September and analysed at the microbiological laboratory at SMI for the presence of E. coli and coliform bacteria by Colilert 18 (IDEXX, USA), intestinal enterococci by Enterolert (IDEXX), (oo)cysts of Giardia spp. and Cryptosporidium spp. according to ISO 15 553:2006, Clostridium perfringens according to ISO/CD 6461-2:2002, somatic coliphages according to ISO 10 705-1:2000, Campylobacter spp. by culture on CCDA agar (in-house method), Salmonella spp. according to ISO 6340:1995, Verotoxin-producing E. coli (VTEC) by PCR screening for vt genes and NoV by semi-nested PCR [Reference Gallimore22]. Additional water samples were collected on 17 September and analysed for presence of NoV and coliphages. Moreover, ice frozen before the outbreak was traced and examined for NoV and coliphages.
Estimate of costs
To estimate the costs related to the outbreak questionnaires were sent out in December 2008 to the municipality of Lilla Edet and other organizations that were included in the outbreak investigation team and to the municipalities of Kungälv and Gothenburg which have their raw water intake located downstream of Lilla Edet and thereby were affected by the outbreak. The questionnaire included questions about costs of labour hours for the management of the outbreak, analysis of human and water samples, telephone conferences, questionnaires that were sent to inhabitants in Lilla Edet, follow-up meeting held in November 2008, and action taken at WTPs downstream of Lilla Edet due to the ongoing outbreak.
Other costs that were estimated included sick leave absence due to gastroenteritis, boiling of drinking water and purchase of bottled water. The calculated cost for boiling drinking water was based on the assumption that 1 litre of water was boiled per day per inhabitant during the 17-day period with the boil water recommendation in place. The electricity consumption for boiling water was assumed to be 0·1 kWh/litre water and the price per kWh was ∼1 Swedish krona (SEK) (∼€0·1). The media reported a large increase in sales of bottled water and the assumption was made that 10% of the inhabitants purchased bottled water corresponding to 10 SEK (∼€1) daily during the 17-day period.
The absence from work or school for persons who became ill with gastroenteritis was assumed to be 4 days which includes 2 days with gastrointestinal symptoms and 2 days after recovery since the CMO consistently urged people to stay at home for 48 h after resolution of symptoms to reduce the risk of secondary transmission. Like many other countries Sweden has paid sick leave. Moreover the parent of a sick child can receive paid leave to care for the child. The cost of sick leave was calculated from statistics over average annual income for the population from the age of 16 years in Lilla Edet [23]. The average annual income with an additional 40% for social benefits was divided by 365 days to represent the cost for working days and days off work. The same cost was applied to individuals aged <16 years assuming the value of their education or that the younger ones had to be cared for at home by an adult (sick child leave).
RESULTS
Epidemiological investigation
Out of the 1199 questionnaires that were sent to randomly selected inhabitants, completed questionnaires were received from 792 (66%) with 2030 personal responses up to 14 October. Another 38 questionnaires that were returned after 14 October were not included in the analysis because of the long time span between the outbreak and the reply; and 369 questionnaires were not returned. For four of the 792 completed questionnaires information about postal codes was missing. The 788 questionnaires, which included information about postal codes, were evenly distributed from the different areas in the municipality (data not shown).
Of the 792 questionnaires, 231 (29·2%) reported at least one case of acute gastroenteritis in the household. Of the 2030 personal responses 379 (18·7%) reported symptoms of acute gastroenteritis. All ages and both sexes were affected. The epidemic curve shows the distribution of the 379 cases by date of onset of symptoms (Fig. 1). The shape of the curve with a steep up slope and a gradual down slope indicates a point-source epidemic in which people were exposed to the same source over a relatively brief period.

Fig. 1. Distribution of 379 cases with acute gastroenteritis by date of onset of symptoms obtained through a questionnaire survey. Black arrow indicates outbreak alert and issuing of boil water recommendation, and white arrow indicates detection of norovirus of genetic diversity in the first analysed stool samples.
The risk of being a case was almost five times higher (OR 4·73, 95% CI 3·53–6·32) for persons in households supplied with water from Lilla Edet WTP vs. persons in households with other drinking water sources (Table 1). More than half of 61 persons in households with other water sources that reported symptoms of acute gastroenteritis had answered in the questionnaire that they had consumed municipal drinking water at nursery school, school, or at their workplace in the municipality. There was a strong correlation between the risk of being sick and the number of glasses of water consumed in households with water supplied by Lilla Edet WTP (Table 2).
Table 1. Exposure to drinking water from Lilla Edet water treatment plant (WTP), or drinking water from other sources, and risk of developing symptoms of acute gastroenteritis

OR, Odds ratio; CI, confidence interval.
Table 2. Correlation of risk of developing symptoms of acute gastroenteritis with number of glasses of drinking water from Lilla Edet water treatment plant consumed per day
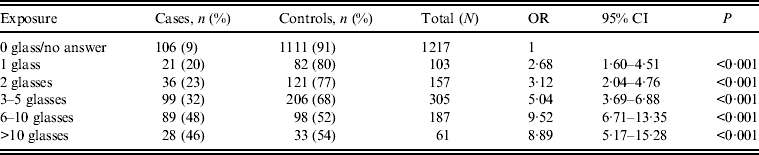
OR, Odds ratio; CI, confidence interval.
From the questionnaire survey it could be calculated that out of the 7500 inhabitant that lived in households supplied by drinking water from Lilla Edet WTP, about 2000 (26·7%) fell ill with acute gastroenteritis. In total, about 2400 (18·5%) of the 13 000 inhabitants in Lilla Edet became sick during 5 to 21 September. Other alternative routes of transmission of NoV, e.g. the possibility of food service at some major local event, were excluded during the outbreak investigation.
Microbiological investigation
NoV was detected in 33/50 stool samples collected from patients with symptoms of acute gastroenteritis. NoV strains of genogroup I (GI) predominated in 31 of these samples and mixed genotypes of GI infections occurred in five samples, as described by Nenonen et al. [Reference Nenonen20]. Adenovirus was detected in one, sapovirus in one and rotavirus in three stool samples. Campylobacter spp. were isolated from two stool samples.
In samples from the drinking water system in Lilla Edet collected on 12 September, E. coli, coliforms, enterococci, Giardia, Cryptosporidium, Clostridium, Campylobacter or NoV could not be detected, while faecal indicator bacteria and coliphages [300 plaque-forming units (p.f.u.)/100 ml] were found in raw water samples from the Göta Älv river. However, somatic coliphages in concentrations between 4 and 42 p.f.u./100 ml were detected in samples from the drinking water system collected on 17 September and in samples from the raw water collected on the same date. Analysis of ice produced before the outbreak was negative for NoV and coliphages.
Estimate of costs
Completed questionnaires were returned from the organizations in the outbreak investigation team and from the municipality of Gothenburg. A total of about 1750 labour hours were related to the management of the outbreak. Twenty percent of these labour hours were reported from the municipality of Lilla Edet and 80% from the other organizations in the outbreak investigation team. The cost for all the labour hours was estimated as SEK 755 000 (∼€75 500) (Table 3). Measures taken at the WTP at Gothenburg, downstream of Lilla Edet, due to the ongoing outbreak were estimated as SEK 282 000 (∼€28 200). Analysis of water samples from Lilla Edet and measures taken at the WTP totalled SEK 150 000 (∼€15 000). The cost of sick leave was calculated as SEK 7 290 000 (∼€729 000). Purchase of bottle water was estimated as SEK 105 000 (∼€10 500) and for boiling of drinking water SEK 8400 (∼€840). Total expenditures arising from the waterborne outbreak were ∼8 700 000 SEK (∼€0·87 million). The cost of sick leave due to acute gastroenteritis represented 84% of the total cost for the outbreak (Table 3).
Table 3. Estimated costs associated with the waterborne outbreak of norovirus in Lilla Edet
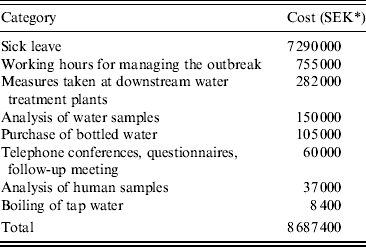
* 1 SEK ∼ €0·1.
DISCUSSION
The questionnaire survey showed that about 2400 inhabitants fell ill during the outbreak. The survey, however, may include some biases. The questionnaires that were sent to randomly selected inhabitants in Lilla Edet included questions about all members in the household, and thereby, persons living in large households may be overrepresented compared to person living in small households. Inhabitants aged >75 years, representing less than 7% of the population, were not included in the survey, unless they lived in a household with younger household members. Thus, the information about persons aged >75 years is limited. The mailed questionnaires, including questions about the period 5–21 September, reached the addressees on 22 September, and at that time, the outbreak was probably well-known since a boil water recommendation was issued on 11 September and the outbreak received intense daily media coverage. This may have influenced the answers in the questionnaire, especially regarding the amount of water consumed. On the other hand, the media attention may have helped the responders to remember details about dates and sickness. Persons who did not live in Lilla Edet, but who worked in that municipality and became ill during the outbreak, were not included in the study and thereby the total number of cases may be underestimated.
The epicurve shows the typical shape of a point-source outbreak (Fig 1). In Figure 1 the epicurve includes all reported cases from the questionnaire survey, and may thereby also include some cases caused by secondary transmission. An epicurve with only the first case in the household included, however, shows the same shape and width, although it is somewhat lower (data not shown). It is not possible to distinguish between cases with different incubation times from exposure of drinking water to development of gastrointestinal symptoms, and cases that were caused by secondary transmission of virus from sick persons. In addition, cases may have had their exposure to drinking water outside the household in other places in the municipality, e.g. at the workplace or in school. Both primary and secondary cases can be considered as a consequence of the contaminated drinking water and should therefore be included as part of the outbreak.
NoV was detected in 33/50 stool samples from patients. Interestingly, these NoVs showed considerable strain diversity [Reference Nenonen20], which in earlier studies has been shown to be associated with outbreaks caused by faecal contamination of water [Reference ter Waarbeek9, Reference Kukkula24, Reference Le25]. In the Lilla Edet outbreak early detection of NoV strain diversity in stool samples strengthened the initial hypothesis that contaminated drinking water was the point source. In addition to the predominant findings of NoV in stool samples, a few other viruses and two Campylobacter spp. were identified in the samples. These latter findings may represent background cases and may not necessarily relate to the outbreak.
Analysis of water samples during the outbreak did not detect NoV in drinking water. The failure to detect NoV in the water sampled during the outbreak might be explained by shortcomings in the technique for identifying NoV in water samples collected at that time [Reference Bosch26]. However, somatic coliphages were detected and this finding suggests the presence of NoV since earlier studies have shown a correlation between the concentration of coliphages and viral contamination [Reference Baggi, Demarta and Peduzzi27, Reference Skraber, Gassilloud and Gantzer28]. Several authors have considered coliphages suitable indicators for the probable presence of enteric viruses [Reference Lucena29–Reference De33].
In Sweden, about half of the inhabitants are served by drinking water obtained from surface water sources such as lakes or watercourses. The Swedish regulations stipulate numbers of microbial barriers dependent on raw water quality. Chlorination that can be counted as a barrier is a common disinfection. In Sweden, there is no lower limit for chlorine residual, only a maximum of 0·4 mg total chlorine per litre drinking water [34]. During the weeks prior to the outbreak, the pre-chlorination of the drinking water at the Lilla Edet WTP had been increased as a preventative measure due to an increased amount of coliforms and E. coli in the Göta Älv river.
As mentioned earlier, there were heavy rains in the area before the outbreak and several combined sewer overflows occurred upstream of Lilla Edet. A turbidity peak was registered on 6 September at monitoring stations downstream of Lilla Edet, suggesting heavy contamination of the river water in Göta Älv, and the levels of E. coli in the river water increased significantly between 5 and 8 September [Reference Heinicke21]. Turbidity and chlorine-demanding solutes from sewage inhibit the disinfection from chlorine [19]. Thus, the waterborne outbreak in Lilla Edet was most probably a consequence of the heavy faecal contamination of the raw water from Göta Älv river [Reference Nenonen20]. The river water, however, is continuously affected by the discharge of microorganism from treated wastewater [Reference Astrom15] and investigations made by Heinicke et al. could not explain why the NoV outbreak in Lilla Edet occurred specifically in September 2008 [Reference Heinicke21].
In the Lilla Edet outbreak the major cost associated with the outbreak was sick leave, representing 84% of the total estimated cost of SEK 8 700 000 (∼€0·87 million). In addition to sick leave, costs for labour hours for management of the outbreak, measures taken at the WTP downstream of Lilla Edet, analysis of stool and water samples, purchase of bottled water and costs for boiling drinking water during the 17-day period of the boil water recommendation were included in the total estimated cost. However, other costs such as expenses for restaurants and other business or possible cost for loss of confidence in the municipal drinking water are difficult to calculate and were not included in the calculations.
The cost for sick leave, SEK 7 290 000 (∼€729 000), is estimated from the reported incidence of acute gastroenteritis during the waterborne outbreak collected through the questionnaire survey. There may be several limitations in this approach. First, the absence for each sick person was assumed to be 4 days, although it might have been either shorter or longer; second, there could have been cases with acute gastroenteritis that were not related to the waterborne outbreak; and third, persons living outside of Lilla Edet and who were exposed to the contaminated drinking water during visits to friends or at their workplaces in the municipality, were not included in the calculations.
We have found few reports on estimates of costs associated with waterborne outbreaks [Reference Baker2, Reference Corso3, Reference Laursen35, Reference Halonen36], which may indicate the difficulties of collecting proper information about costs. It may also reflect the difficulties in deciding on which costs actually should be included in the estimates and in finding suitable methods for collection of the data. Sick leave is a common parameter that is frequently included in estimates of outbreak costs. However, the method for collection of this information varies between different outbreak reports. For example, in a report from a Danish waterborne outbreak [Reference Laursen35] information about sick leave due to gastroenteritis was obtained through a structured questionnaire that was sent to all households that were supplied by the actual waterworks. These authors reported a cost figure for sick leave without explaining how they had made the calculations. In a waterborne outbreak in Finland [Reference Halonen36] information about costs for sick leave in public sector employees was obtained from the employers’ registers that gave a very precise cost figure. However, by using this method information about sick leave for persons working outside the public sector was unknown. Moreover, the diagnoses that caused the sick leave were unknown since the employers’ registers did not include this information.
Cases that stand apart (called ‘outliers’) may provide important information during outbreak investigations to reject or strengthen a hypothesis regarding the source of the outbreak. In this outbreak, the sick athletes strengthened the early suspicion of drinking water being the point source since they had made a very short visit to Lilla Edet and had consumed only drinking water from the Lilla Edet WTP and had not consumed any food.
The problems of identifying an ongoing waterborne outbreak may partially be explained by the fact that attack rate, symptoms and incubation period differ between different pathogens. Outbreaks of waterborne NoV, a pathogen which causes a short duration of illness with few affected individuals seeking healthcare, which would provide the opportunity for stool sampling for analysis, may fail to be recognized for a longer period of time than outbreaks caused by other pathogens. In the outbreak in Lilla Edet, it was a nurse at the PHC who, via a phone call to the CMO, gave the first alert that several persons in the municipality had fallen ill with gastroenteritis. This emphasizes the importance of a well-established cooperation between healthcare and the CMO. The promptly issued boil water recommendation may have reduced the number of persons that fell ill during this outbreak, as indicated by the down slope of the epicurve (Fig. 1). Similarly, a well-established liaison between laboratory, PHC and CMO provides for adequate patient sampling and efficient routes of communication when an urgent response is required. The rapid detection of NoV of genetic diversity in the first analysed stool samples was an indicator for a point source of faecal contamination of the drinking water system, and thereby strengthened the initial hypothesis of a waterborne outbreak. This hypothesis was later confirmed by the results from the epidemiological investigation.
ACKNOWLEDGEMENTS
We thank all the persons who were involved in the management of the outbreak, especially Jill Johansson, Birgitta Loewen and Margareta Olsson at the local office Uddevalla, Department of Communicable Disease Control and Prevention, Region Västra Götaland, for their valuable work with data collection and documentation during the outbreak.
Y. Andersson has now retired from the Swedish Institute for Communicable Disease Control, Solna, Sweden.
DECLARATION OF INTEREST
None.






