INTRODUCTION
Zoonoses are diseases that can be transmitted from animals to humans and are public health threats worldwide. Zoonoses caused by Gram-negative, obligate intracellular bacteria, members of the genera Rickettsia and Coxiella, are diseases of global importance.
Mediterranean spotted fever (MSF) is an acute, febrile, tick-transmitted rickettsiosis caused by Rickettsia conorii, which is endemic in Mediterranean countries, Africa and southern Asia [Reference Raoult and Roux1–Reference Blanco and Oteo4]. The major vector of the disease is the brown dog tick Rhipicephalus sanguineus [Reference Raoult and Roux1]. The first well-documented, serologically confirmed cases of MSF in Croatia were reported among inhabitants of Split and its adjacent suburban area in 1982 [Reference Punda5].
Murine typhus (MT) also known as endemic typhus, is an infectious disease caused by Rickettsia typhi. R. typhi is maintained in rats (Rattus norvegicus and Rattus rattus) and transmitted to humans through contamination of the skin, conjunctivae, or respiratory tract by the faeces of the rat flea Xenopsylla cheopis [Reference Raoult and Roux1–Reference Blanco and Oteo4, Reference Azad6]. The disease has a high prevalence throughout the world, especially in warm areas, and particularly in ports and coastal regions infested with rats and fleas [Reference Raoult and Roux1–Reference Blanco and Oteo4, Reference Azad6–Reference Gikas9]. MT may also be associated with European [Reference Parola and Raoult3, Reference Parola10] and American [Reference Walker2, Reference McDonald, MacLean and McDade11] travellers returning from countries or regions where the disease is prevalent. In southern Croatia MT has been recognized since 1942 [Reference Urlić12].
Q fever is a zoonosis caused by Coxiella burnetii, a strictly intracellular bacterium [Reference Maurin and Raoult13]. The disease occurs in various geographic regions and climate zones world-wide [Reference Maurin and Raoult13]. The principal reservoirs of C. burnetii are ticks, cattle, sheep and goats [Reference Maurin and Raoult13]. C. burnetii is highly infective in aerosols [Reference Maurin and Raoult13, Reference Raoult, Marrie and Mege14]. In humans, most infections occur after inhalation of contaminated dust or aerosols generated by infected domestic animals, mainly sheep and goats. Q fever is symptomatic in less than 50% of cases, is clinically non-specific, and may manifest in an acute or chronic form [Reference Maurin and Raoult13]. Because of its high infectivity and resistance, C. burnetii has been considered as an agent that potentially could be used as a biological weapon [Reference Raoult, Marrie and Mege14]. In Croatia, Q fever was first recognized in 1949 in the northern part of the country [Reference Mihaljević15].
In this paper we describe the incidence, clinical and epidemiological features of these three zoonoses (rickettsioses) recognized in hospitalized patients in southern Croatia. This study summarizes data collected over 21 years.
PATIENTS AND METHODS
Study area
The study was performed in Split-Dalmatia County, in the south of Croatia, on the Adriatic coast (latitude 43° 02′ N to 43° 56′ N, longitude 16° 04′ E to 17° 18′ E). The county is the second largest in Croatia, with a total land (mainland and islands) area of 4572 km2. The islands, and coastal belt, situated beneath steep slopes of the Dinaric Alps, are characterized by a Mediterranean climate with mild and rainy winters, dry summers and a mean annual air temperature of 16·3°C. The northern, mountainous part is situated in the hinterland, at 380–1000 m above sea level, and has a sub-Mediterranean climate (mean annual air temperature 12·6°C). According to the 1991 census, the county had a population of 474 019, with a 1:1·03 male:female ratio [16].
Two areas in the study region were differentiated according to geographic characteristics and economic activities: (1) littoral – including towns and their suburbs, situated on the coast, their economy depending on industry (shipbuilding) and tourism; and islands (Vis, Hvar, Brač and Šolta), where the inhabitants are mostly engaged in tourism, fisheries, and grape and olive cultivation; and (2) hinterland – a mountainous area with fertile valleys accommodating small towns and villages, where the inhabitants mainly work in agriculture and stock breeding (mostly sheep and goats).
The study was retrospective by design for the period 1982–1990, using data from serological testing and medical records of cases treated for MSF, MT, and acute Q fever at Split University Hospital, the only hospital in the county. In 1991 we started the prospective study in order to improve our knowledge of and explore the clinical and epidemiological features of these zoonoses. The following data were recorded: (a) demographic and epidemiological: age, gender, place of residence, contact with animals and arthropods; (b) clinical signs and symptoms: elevated body temperature, headache, arthralgia, myalgia, malaise, rash: (c) data on therapy and treatment outcome.
Patients
The study included Split-Dalmatia County residents who were hospitalized at the Department of Infectious Diseases, Split University Hospital due to one of the three diseases. Patients were identified according to clinical features and diagnosed by serological methods. A standardized questionnaire was used to collect demographic and clinical information.
MSF was suspected in patients presenting with two of the following symptoms: fever >38°C, headache, eschar (tache noire), and a macular or maculopapular rash [Reference LaScola and Raoult17]. MT was suspected in patients with fever >38°C, and/or headache, and/or rash with centripetal distribution [Reference LaScola and Raoult17]. Q fever was suspected in patients clinically presenting with fever >38°C, and headache, with or without pneumonia or hepatitis [Reference Fournier, Marrie and Raoult18].
Serology
For each patient, an acute-phase serum specimen was obtained; a convalescent-phase serum specimen was obtained at least 2 weeks later. Serum samples were tested prior to 1988 by complement fixation test. After 1988, the indirect immunofluorescence assay (IFA) using commercially available antigens (Rickettsia conorii – Spot IF, Rickettsia mooseri – Spot IF, and Coxiella burnetii – Spot IF; bioMérieux, Marcy l'Etoile, France) was performed to detect the titres of IgM and IgG in patient sera. A diagnosis of each zoonosis was considered established when characteristic symptoms were present and associated with either a seroconversion or at least a fourfold increase in antibody titres [Reference LaScola and Raoult17, Reference Fournier, Marrie and Raoult18].
Statistical analysis
Results were analysed statistically using Statistica 6.0 software (StatSoft Inc., Tulsa, OK, USA). Incidences were calculated from the 1991 Croatian census data [16]. The frequency of cases were compared for the coastal and hinterland areas of the study region. The χ2 test was used to compare qualitative data frequencies. To assess seasonality, the frequency of cases by month of onset was examined. Differences were considered statistically significant at P<0·05.
RESULTS
Mediteranean spotted fever
From 1982 to 2002, 126 patients were diagnosed as having MSF and hospitalized at the Split University Hospital. The signs and symptoms of patients with confirmed MSF included fever and malaise (100%), rash (97·2%), headache (66·3%), and arthralgia/myalgia (43·6%). Eschar (tache noire) was found in 80 (63·5%) patients. No deaths were recorded.
Seventy-nine (62·7%) of the patients were male (incidence rate 33·88/100 000 inhabitants), and 47 (37·3%) were female (incidence 19·52/100 000) (Table 1). The male:female ratio was 1·7:1 with significant difference (P=0·0024). The mean age of patients was 33·3 years (s.d.±21·9; range 11 months to 79 years). Age-specific cumulative incidence data indicated the peak (23·8%) at age 0–10 years (incidence 52·8/100 000 inhabitants (Table 2). Fifty-two (41·3%) of the patients were aged <20 years. The lowest incidence of MSF was in the 21–30 years age group (7·5/100 000 inhabitants). Although 90 (71·4%) patients originated from the islands and coastal area, and 36 (28·6%) from the hinterland area, there was no statistical difference in the incidence rate/100 000 inhabitants (P=0·59). The annual incidence of MSF ranged from 1 (2000) to 15 (1991–1992) cases per year (an average of 1·27 cases/100 000 population per year). Regarding the months of observation (Fig. 1), most cases (81·7%) occurred from July to September, with a peak incidence in August (42·1%). Ninty-nine patients (78·6%) had a history of contact with dogs. Seventy-eight (61·9%) patients recalled a tick bite or removal of a tick before the disease developed.
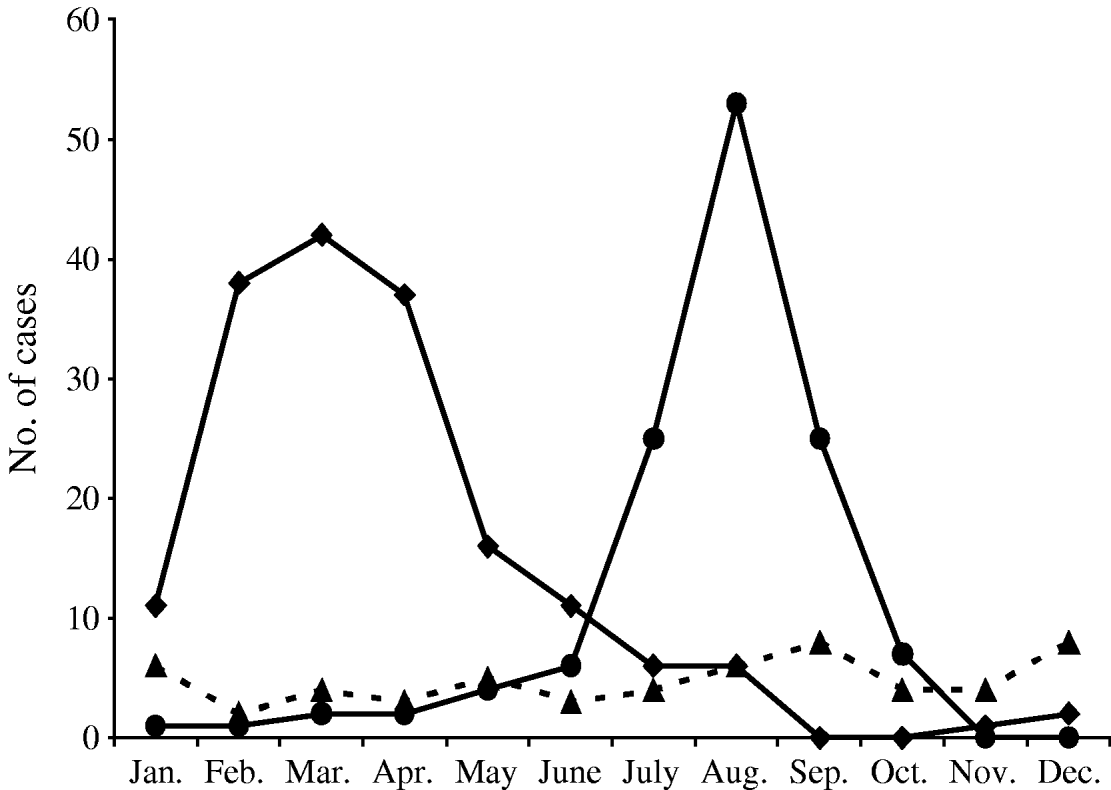
Fig. 1. Seasonal distributions of Q fever (–◆–), Mediterranean spotted fever (–●–) and murine typhus (- -▲- -) cases, Split-Dalmatia County, Croatia, 1982–2002.
Table 1. Gender distribution of Mediterranean spotted fever (MSF), murine typhus (MT) and Q fever cases in Split-Dalmatia County, Croatia, 1982–2002
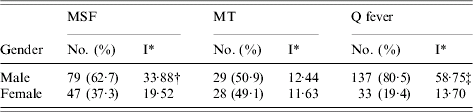
* I, Cumulative incidence rate per 100 000 inhabitants.
† χ2=9·2, P=0·0024; ‡ χ2=67·0, P<0·0001; other differences were not significant.
Table 2. Distribution of Mediterranean spotted fever (MSF), murine typhus (MT) and Q fever cases according to age of patients, Split-Dalmatia County, Croatia, 1982–2002


* I, Cumulative incidence rate per 100 000 inhabitants.
Murine typhus
A total of 57 patients were diagnosed as having MT. The signs and symptoms of patients with serologically confirmed MT included fever (100%), headache (75·0%), rash (70·8%), malaise (53·2%), and arthralgia/myalgia (43·6%). Rash was distributed over the trunk and limbs, sparing the face, palms and soles. None of the patients died.
There were no significant differences in the incidence rate of MT according to gender. Twenty-nine (50·9%) of the patients were male and 28 were female (P=0·80) (Table 1). MT occurred in patients of all age groups. The mean age of patients was 37·9 years (s.d.±15·3; range 7–72 years). More than half of the cases (39, 68·4%) were in the 21–50 years age group (Table 2). Thirty-eight (66·7%, incidence 10·9/100 000) patients originated from islands and coastal area, and 19 (33·3%, incidence 15·15/100 000) from hinterland areas but there was no statistical difference in the incidence rate/100 000 inhabitants (P=0·24). The annual incidence of MT ranged from 0 (1984) to 11 (1991) cases per year (an average of 0·57 cases/100 000 population per year). Regarding the months of observation (Fig. 1) cases of MT were observed all year round (from two cases in February to eight cases in September and December, respectively). Twenty-six patients (45·6%) had a history of regular contact with domestic animals. Twenty-five (43·9%) patients reported observing rats and mice around houses. No patients mentioned flea bites or contact with ticks.
Q fever
During 1982–2002, acute Q fever was laboratory-confirmed in 170 patients hospitalized at the Department of Infectious Diseases, Split University Hospital. The signs and symptoms of patients with confirmed Q fever included fever (98·5%), headache (86·6%), malaise (74·7%), coughing (69·6%), arthralgia/myalgia (50·5%) and rash (2·6%). Clinically, acute Q fever most commonly presented with both pneumonia and hepatitis (60·0%), followed by pneumonia (25·8%), hepatitis (9·0%), and non-specific febrile illnesses (5·2%). Gender distribution of Q fever cases indicated a male predominance (χ2=67·0, P<0·0001) (Table 1). Of the 170 cases, 137 (80·5%) were males (incidence rate 58·75/100 000) and 33 were females (incidence rate 13·70/100 000), yielding a male:female ratio of 4·1:1. Data on age distribution of acute Q fever cases are presented in Table 2. The mean age was 34 years (s.d.±12·8, range 4–76). The highest rates of acute Q fever was recorded in the 21–30, 31–40 and 41–50 years age groups, respectively. The 21–50 years age group accounted for 81·8% of the 170 cases. Three cases were recorded in each of the 0–10 and >71 years age groups. Five familial clusters or outbreaks of the disease were recorded. During the study period, the number of new cases varied from year to year. From 1982 to 2002, a peak incidence of 22 was noted in 1996; with the exception of this one year, cases ranged from 0 to 17 patients (an average of 1·71 cases/100 000 population per year). We recorded 145 acute Q fever cases during the 1992–2002 period, a significant increase over the 25 cases hospitalized in the 1982–1991 period (χ2=84·7, P<0·001) (Fig. 2). The monthly distribution of acute Q fever cases indicates a spring clustering pattern. Most cases (117, 68·8%) presented between February and April, the greatest number being recorded in March (24·7%) (Fig. 1). Q fever cases significantly predominated in the hinterland area (χ2=284, P<0·0001), with predominantly rural settings and inhabitants mostly engaged in sheep and goat breeding. Only 28 (16·5%, incidence 8·03/100 000) cases of Q fever recorded during the study period were from coastal areas. None of the acute Q fever cases originated from the study area islands. Contact with domestic animals on a daily basis was identified in 143 (84·1%) and contact only with sheep in 107 (62·9%) cases. Three (1·8%) patients recalled contact with ticks, and eight (4·7%) observed rats and mice in the vicinity of houses. All patients recovered completely, with no complications within a 1-year follow-up.
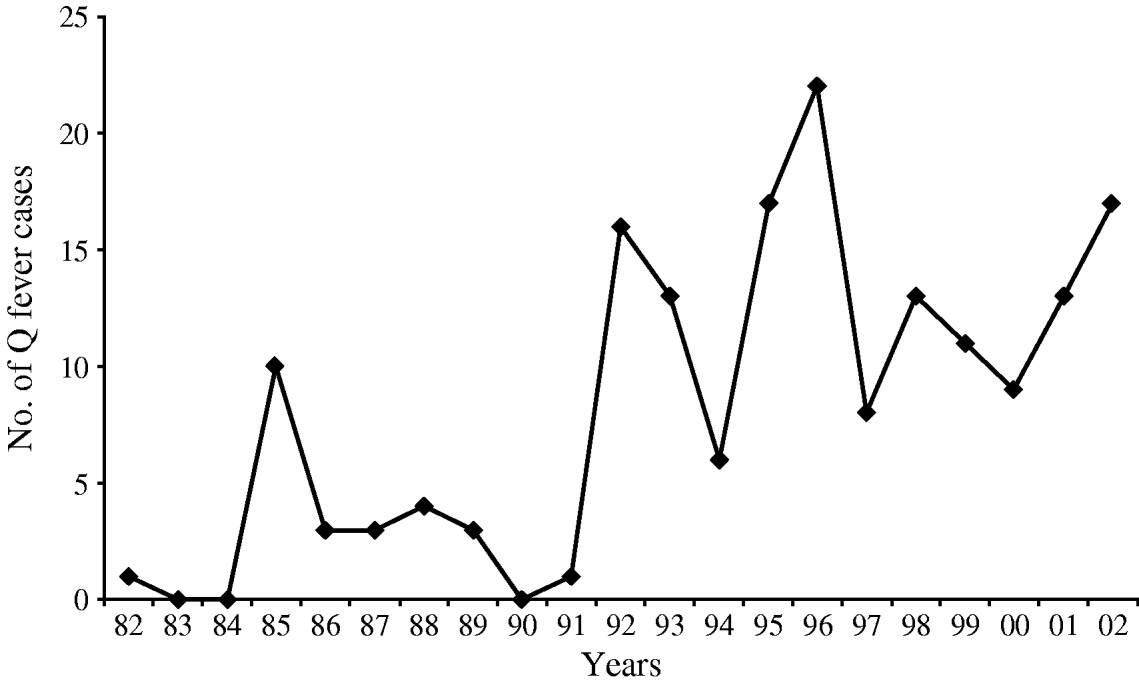
Fig. 2. Annual distribution of 170 cases of acute Q fever in Split-Dalmatia County, Croatia, 1982–2002.
DISCUSSION
Our study confirms the widespread distribution of R. conorii, R. typhi and C. burnetii in the area of Split-Dalmatia County, Croatia. Patients were identified according to clinical features and diagnosed by serological methods. For serological diagnosis the use of an appropriate cut-off titre is a critical issue and this titre should be established and should vary according to the incidence of disease and the distribution of the pathogen in the area studied [Reference LaScola and Raoult17, Reference Fournier, Marrie and Raoult18]. Since we did not have a predetermined cut-off titre, we considered that the diagnosis of the zoonoses studied was confirmed when a compatible clinical picture was present together with a seroconversion or when a fourfold or higher increase in titres between acute and convalescent sera was found. This approach prevented false diagnoses, although we might have missed a few cases.
During this study 126 patients met clinical and serological criteria for MSF. R. conorii was used as the sole antigen in the immonofluorescent serological assay. Although one of the major limitations of serological testing for diagnosis of rickettsioses is the cross-reactivity between different Rickettsia species, the association of R. conorii with the spotted fever cases was considered appropriate, especially because R. conorii (Malish strain) was isolated from the blood of one of our patients [Reference Sardelić19]. Among the demographic parameters of our patients, gender was important. Males were more frequently diagnosed as having MSF than were females, as stated previously in other studies of MSF [Reference Raoult20–Reference Font-Creus23]. Regarding the age of patients, all age groups were affected, with a peak in the 0–10 years age group. This is similar to what was found for MSF in other studies [Reference Raoult21–Reference Font-Creus23]. We found a clear predominance of MSF cases during the summer period when the tick vectors are most active [Reference Parola, Paddock and Raoult24]. This seasonal distribution of MSF, showing a peak in August, is in agreement with results of other studies in Mediterranean countries [Reference Blanco and Oteo4, Reference Raoult20–Reference Raoult26]. With regard to annual incidence, there was yearly variation in the number of cases, as has been shown in other studies in Mediterranean countries [Reference Raoult and Roux1, Reference Raoult21, Reference Segura-Porta22]. The highest incidence of 15 MSF cases was detected in 1991 and 1992. Although in the 1970s as well as in the early 1980s an increasing incidence of this disease was reported in many countries [Reference Segura-Porta22, Reference Font-Creus23, Reference Mansueto, Tringali and Walker27] no regular increase in the incidence of clinical cases was noted in the last years in our area, as has also been reported in other areas [Reference Raoult20, Reference Herrero-Herrero25]. We found no difference in the incidence of MSF regarding residence area. In contrast to our study, studies in France [Reference Raoult20, Reference Raoult26] and Spain [Reference Segura-Porta22, Reference Font-Creus23] report a predominantly urban origin of cases. Ninety-nine (78·6%) of our patients had a history of contact with dogs, while in other endemic Mediterranean areas contact with dogs was confirmed in 79–92% of MSF cases [Reference Raoult20–Reference Font-Creus23, Reference Herrero-Herrero25]. Contact with ticks was recorded in 61·9% of our patients, which is higher than the finding in France, where a history of tick contact was found in 28–46·6% of the patients [Reference Raoult20, Reference Raoult21, Reference Raoult26]. In the study in Salamanca, Spain, tick contact was reported by 98·7% of the patients [Reference Herrero-Herrero25]. Eschar, at the site of the tick bite, was found in 63·5% of our patients. The rate of its presence in other studies has been recorded in 50–89% of MSF cases [Reference Raoult20–Reference Font-Creus23, Reference Herrero-Herrero25]. Although MSF has been considered to be a mild disease, malignant forms have been reported, mostly among elderly patients or those with underlying diseases and the mortality rate may reach 2·5% [Reference Raoult and Roux1, Reference Parola and Raoult3, Reference Raoult20]. Although our study included only patients whose illnesses were severe enough to warrant hospitalization, there were no fatal outcomes. This could be because most of our patients (41·3%) were previously healthy subjects aged <20 years. Because a typical clinical picture of MSF with a history of contact with dogs or ticks, was not regularly present the disease is probably often underdiagnosed due to lack of awareness. The clinical features of MT observed in our study cohort were consistent with those reported by other studies [Reference Walker2, Reference Parola and Raoult3, Reference Azad6–Reference Parola10, Reference Bernabeu-Wittel28, Reference Hernandez-Cabrera29], with fever, headache, malaise and arthromyalgia being the most frequent signs and symptoms. The frequency of rash in our patients was similar to that reported by Tselentis et al. [Reference Tselentis8], higher than that reported by Dumler et al. [Reference Dumler, Taylor and Walker7] and Bernabeu et al. [Reference Bernabeu-Wittel28] and lower than that reported by Gikas et al. [Reference Gikas9]. In our study MT cases were distributed in practically all age groups and living areas, probably reflecting the broad and persistent presence of infected peridomestic rat reservoirs. The incidence of cases according to gender does not differ significantly, indicating that the degree of exposure for men and women in this area may be very similar. In contrast with our study some other studies reported a predominance of female [Reference Dumler, Taylor and Walker7] or of male cases [Reference Tselentis8, Reference Gikas9, Reference Bernabeu-Wittel28, Reference Hernandez-Cabrera29]. Thus, it is apparent that a great variation exists in the occurrence of MT among males and females in different geographical regions that is probably related to risk practices within the study population. In this study MT cases occurred throughout the entire year, which is different from observations in other studies, in which prominent seasonal distribution of human cases was observed [Reference Blanco and Oteo4, Reference Azad6–Reference Gikas9, Reference Hernandez-Cabrera29]. Consistent with other reports [Reference Raoult and Roux1–Reference Parola and Raoult3, Reference Dumler, Taylor and Walker7] less than half (43·9%) of our cases reported observing rats and mice in and around houses, while in a study in Greece contact with rats was noted by 54% of the patients [Reference Gikas9]. None of our patients mentioned flea bites, which is in agreement with other reports [Reference Walker2, Reference Azad6]. The incidence of MT may be higher than we describe in this report, since less severe and more non-specific clinical manifestation of MT frequently go unrecognized, and several studies indicate that subclinical infection with R. typhi is common worldwide [Reference Raoult and Roux1–Reference Parola and Raoult3, Reference Azad6]. In addition, since the cross-reaction between R. typhi and R. felis is known, we can speculate that some MT-like illnesses may have been caused by R. felis [Reference Raoult and Roux1, Reference Parola and Raoult3, Reference Blanco and Oteo4].
Of the 170 cases of acute Q fever diagnosed, 83·5% had been infected in rural hinterland areas of the study region. None of the cases were from the islands in the region, probably because people on those islands are mostly engaged in tourism, fishery and plant cultivation. In this study, fever, headache and malaise were the most common signs and symptoms. Clinically, Q fever most commonly presented with pneumonia and hepatitis. Pneumonia was observed in 85·8% and hepatitis in 69·0% of these patients. Pneumonia was the principal clinical presentation in Q fever epidemics in Switzerland [Reference Dupuis30], some regions of Spain [Reference Tellez31, Reference Sobradillo32] and on the island of Crete, Greece [Reference Tselentis33], whereas hepatitis was more frequently recorded in France [Reference Raoult34], and California, USA [Reference Clark35]. Febrile syndrome is the most frequently described form of acute Q fever in Australia [Reference Derrick36]. Differences in the clinical presentation of Q fever in various parts of the world may be due to the varying virulence of particular C. burnetii strains, host factors, inoculation dose, and route of infection (aerosol or ingestion of contaminated food) [Reference Maurin and Raoult13, Reference Raoult, Marrie and Mege14, Reference Raoult34]. In France, the predominance of the hepatic form of Q fever in rural areas has been associated with ingestion of raw milk and fresh goats' cheese [Reference Maurin and Raoult13, Reference Raoult34]. In our study, all cases presenting with hepatitis denied using raw milk. In the present study, rash was recorded in 2·6% of cases with acute Q fever, which is lower than generally reported in the literature, where rash is found in 5–21% of Q fever cases [Reference Tselentis33, Reference Raoult34]. Although Q fever generally is considered a mild disease [Reference Maurin and Raoult13, Reference Raoult, Marrie and Mege14], fatal outcomes have been reported [Reference Maurin and Raoult13, Reference Raoult34]. In our study, all cases with acute Q fever recovered completely.
Our study showed that the occurrence of Q fever varies according to age and gender. Its incidence in males was 4·1-fold higher than in women. All studies of Q fever in adults have found it to be more common in males than in females, the male:female attack rate ratio generally ranging from 2·3:1 to 2·96:1 [Reference Raoult and Roux1, Reference Raoult34, Reference Tissot-Dupont37]. A higher incidence of Q fever in males may be explained by sex hormones as well as by males' greater occupational exposure to C. burnetii [Reference Maurin and Raoult13, Reference Raoult, Marrie and Mege14, Reference Raoult34, Reference Maltezou and Raoult38]. Age distribution of Q fever cases correlates with activities of individuals. In our study, the highest rate of infection was in the 21–50 years age group, which is consistent with other reports [Reference Maurin and Raoult13, Reference Raoult, Marrie and Mege14, Reference Tselentis33, Reference Raoult34]. This may be explained by intensive occupational activities with higher exposure of people in these age groups to sources of C. burnetii infection [Reference Maurin and Raoult13, Reference Raoult, Marrie and Mege14, Reference Raoult34]. Q fever rarely has been described in children, because in children C. burnetii infection is unobserved or causes a milder illness than it does in adults [Reference Maltezou and Raoult38]. In our study, only three Q fever case-patients were <10 years old, with pneumonia in two and meningitis in one. Risk factors for Q fever include staying in a rural area and contact with livestock [Reference Maurin and Raoult13, Reference Raoult34, Reference Hellenbrand, Breuer and Petersen39, Reference McQuiston and Childs40]. This is supported by the results obtained in our study, in which up to 83·5% of affected individuals were residents of rural areas, and contact with livestock occurred in 84·1% of the cases. Results of the present study suggest that sheep are the most likely sources of C. burnetii infection. In Croatia, lambing occurs during winter and early spring. More than half (68·8%) the Q fever cases were registered in the period February to April, coinciding with the time of lambing and more intensive contact between humans and livestock. Sheep have been identified as the most common source of both sporadic infections and outbreaks of Q fever in many other European countries [Reference Maurin and Raoult13, Reference Tissot-Dupont37–Reference Hellenbrand, Breuer and Petersen39] and in the United States [40]. In southern Croatia, the number of acute Q fever cases increased considerably beginning in 1992. Although this increase may in part have been the result of the disease being more frequently considered for patients presenting to the hospital as well as to better recognition and improved diagnosis, the increase in the number of outbreaks implies a true increase in its occurrence. One other possible reason for such a trend may be the changes in economic situation in Croatia, forcing people to rely more on stock breeding, especially sheep breeding.
This study reports the clinical and epidemiological features of three bacterial zoonoses in southern Croatia during 21 years and provides further evidence that the epidemiologies of those zoonoses are different. We conclude that in the region of Split-Dalmatia County, Croatia there are three endemic zoonotic diseases which may be often underdiagnosed. Of the three zoonoses studied, Q fever was the most frequent, indicating that C. burnetii is endemic, especially in the northern rural part of the area. Since our study included only hospitalized patients, further studies are warranted to determine the true incidence rate of the zoonoses studied in the area.
ACKNOWLEDGEMENTS
This work was supported by grants (No. 0126005 and No. 216-0481153-1148) from the Ministry of Science, Education and Technology of the Republic of Croatia. We thank the staff members of the Department of Clinical Microbiology and the Department for Infectious Diseases of Split University Hospital for their assistance.
DECLARATION OF INTEREST
None.






