INTRODUCTION
Campylobacter spp. are a commonly reported cause of infectious gastroenteritis in developed countries. Whilst the incidence of Campylobacter infection in England and Wales has declined in recent years since peaking in 2000, the disease still represents a significant source of morbidity, with over 46 000 laboratory-confirmed cases reported annually [1]. Infection with Campylobacter spp. can manifest across a wide clinical spectrum, from asymptomatic carriage to symptoms indicative of appendicitis [Reference Blaser, Mandell, Bennett and Dolin2]. This, coupled with the fastidious nature of the microorganisms [Reference Vandenberg, Skirrow, Butzler, Borriello, Murray and Funke3], results in laboratory-confirmed cases representing the tip of the infection iceberg [Reference Wheeler4].
Our understanding of the epidemiology of human Campylobacter infection in developed countries is derived mainly from the investigation of outbreaks and from case-control studies of sporadic cases [Reference O'Brien5]. However, the infective dose for Campylobacter infection is low [6, Reference Black7] and the incubation period long and variable [6], meaning that accurately establishing exposure in cases and comparing this with exposure in others is problematic. This, exacerbated by the relatively poor routine follow-up of sporadic cases of human Campylobacter infection at the local level [Reference Rooney8] and the lack of suitable laboratory subtyping methods applicable to all isolates, means that outbreaks of Campylobacter infection are rarely identified [Reference Pebody, Ryan and Wall9, Reference Frost, Gillespie and O'Brien10].
The biases associated with case-control studies are numerous and described elsewhere [Reference McCarthy and Giesecke11]. They include selection bias when the probability of including cases (and/or controls) is associated with the exposure under investigation; and information bias (both recall and observer bias). It is perhaps for these reasons that risk factors for Campylobacter infection identified through case-control studies consistently fail to account for the majority of cases exposed in those studies [Reference Kapperud12–Reference Schonberg-Norio18]. An additional factor which might have reduced the usefulness of case-control studies on Campylobacter infection is that numerous variables, from different transmission routes and various points on the causal pathway, are often considered together. In doing so, bias will often be introduced by inappropriately adjusting for factors that are on the causal pathway. The development of conceptual frameworks for analysis has been suggested as a method of overcoming this [Reference Victora19]. Similarly, studies can be restricted to distinct population groups that might be at particular risk. Both methods, however, require prior knowledge of the social and biological determinants of disease.
The Campylobacter Sentinel Surveillance Scheme was a population-based surveillance scheme for Campylobacter infection in England and Wales which aimed to generate new hypotheses for infection [Reference Gillespie20]. It ran from May 2000 to April 2003 inclusively – a period which coincided with the 10-year Census in the United Kingdom in 2001. This provided a valuable opportunity to compare the demographic characteristics of cases of Campylobacter infection with that of the population from which they arose, with an aim of identifying those demographic subgroups in England and Wales at greatest risk of Campylobacter infection.
METHODS
The Campylobacter Sentinel Surveillance Scheme comprised 22 health authorities from all National Health Service regions across England and Wales. Participating laboratories within the health authority catchment areas referred Campylobacter isolates from all laboratory-confirmed cases to the Public Health Laboratory Service (PHLS) Campylobacter Reference Unit (CRU) for further characterization. A standard, structured clinical, demographic and exposure questionnaire was administered by post or by telephone concurrently to all cases by local public health and environmental health practitioners as part of their routine investigations. Completed questionnaires were forwarded to the Public Health Laboratory Service Communicable Disease Surveillance Centre (now the Health Protection Agency Centre for Infections). Electronic microbiological and epidemiological data were reconciled using patients' surnames and dates of birth.
Data classification was undertaken using Epi-Info version 6.04d [Reference Dean21] and Microsoft Access (Microsoft Corporation, Redmond, WA, USA). Patients' descriptions of their ethnic origin were coded according to the United Kingdom 2001 Census [22]. The occupation descriptions provided by patients of working age (16–74 years) were coded by two contributors (I. A. G., C. P.) according to Standard Occupational Classification [23]. National Statistics Socioeconomic Classification (NS-SEC) was then derived using the self-coded simplified method [24], which was subsequently grouped into analytical class.
In early 2001, health authorities in England and Wales were replaced by Primary Care Trusts (PCTs) and Strategic Health Authorities. Accordingly, age, gender, ethnicity and socioeconomic denominator data for the PCTs which constituted the sentinel health authorities were obtained from Office for National Statistics Standard Tables for health areas. Denominator data for 2001 were used as an approximation of the sentinel population over the entire study period.
Statistical analysis was undertaken using Microsoft Excel (Microsoft Corporation) and Stata version 8.2 [25]. Analysis was restricted to cases that had not travelled abroad in the 2 weeks before illness. Estimates of incidence (cases/100 000 population per year unless stated otherwise) and relative risk (RR), with accompanying 95% confidence intervals (CI) and significance tests were calculated. Changes in proportion for categorical variables were assessed using the χ2 test for trend.
RESULTS
Between 1 May 2000 and 30 April 2003, questionnaires were received for 20 387 of the 28 510 human Campylobacter isolates referred to the PHLS CRU (response rate 72%). Of these, 4109 cases (20%) reported recent foreign travel and a further 371 cases (2%) did not report their travel status. These cases were excluded, leaving 15 907 United Kingdom-acquired cases of Campylobacter infection from a population of 11 281 065 – an indigenous annual incidence of 47·0 cases/100 000 per year (95% CI 46·3–47·7).
Gender and age
The gender of all cases was known and cases were distributed equally across both genders (7965/15 907 male cases; 50%). However, the incidence in males was slightly higher than in females (risk ratio 1·06, 95% CI 1·03–1·10, P=0·0001). Patients' ages were available for 15 855/15 907 cases (99·7%). Overall, incidence was highest in infants (⩽1 year 120·1, 95% CI 112·6–128·0). It decreased for ages 2 to 13 years (from 74·8 to 15·8, χ2 for trend 263·1, P<0·001). The incidence then increased for ages 14 to 22 years (from 16·9 to 56·3, χ2 for trend 223·3, P<0·001) and remained relatively stable for ages 22 to 69 years (52·7, 95% CI 51·7–53·7), before declining for ages ⩾70 years (from 48·1 to 29·0, χ2 for trend 85·7, P<0·001).
The incidence in male and females, and the male to female relative risk of infection for all ages is shown in Figure 1. Overall, incidence in males was higher than in females from birth until the late teens (0–17 years, RR 1·54, 95% CI 1·43–1·66, P<0·001). This effect was observed consistently throughout this age group and was especially marked from 13 to 15 years (RR 2·48, 95% CI 1·85–3·30, P<0·001). Incidence in females was lowest at 14 years but increased rapidly from this age to 22 years (from 7·3 to 63·4, χ2 for trend 219·0, P<0·001), exceeding that in males at 18 years. Although not significant on a year-on-year basis, incidence in females was higher than in males from 20 to 36 years (RR 1·21, 95% CI 1·14–1·29, P<0·001). Incidence varied from 50 years onwards, but was overall higher in males than in females in this age group (RR 1·12, 95% CI 1·06–1·18, P<0·0001).
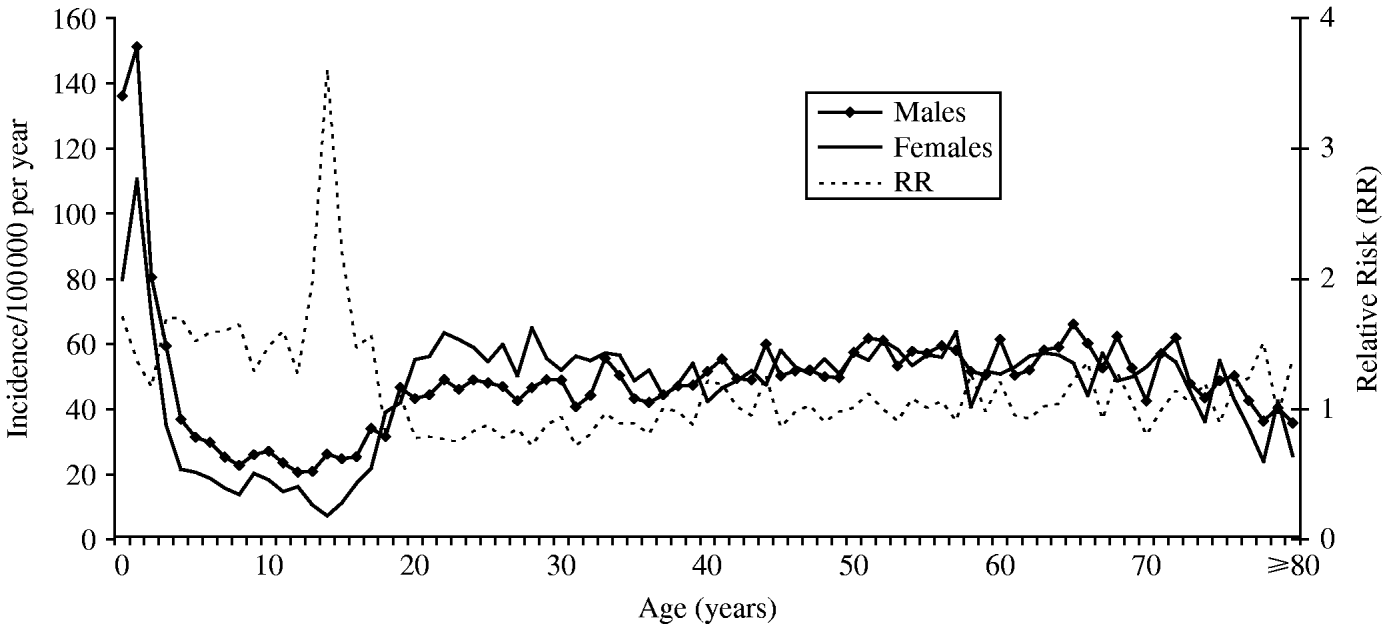
Fig. 1. The incidence by age of Campylobacter infection in males and females, and the male to female relative risk, in the Campylobacter Sentinel Surveillance Scheme population (n=15 855), England and Wales, May 2000 to April 2003.
Ethnicity
Accurate descriptions of ethnic origin were provided by 12 970/15 907 cases (80·4%). The incidence in the resident Pakistani population was higher than in the resident white population (RR 1·14, 95% CI 1·03–1·26, P=0·01), which in turn was higher than that of the resident Indian (RR 2·74, 95% CI 2·30–3·27, P<0·001), Bangladeshi (RR 2·58, 95% CI 1·80–3·69, P<0·001), Black (RR 2·49, 95% CI 2·07–3·01, P<0·001) and Chinese (RR 1·85, 95% CI 1·31–2·60, P<0·01) communities.
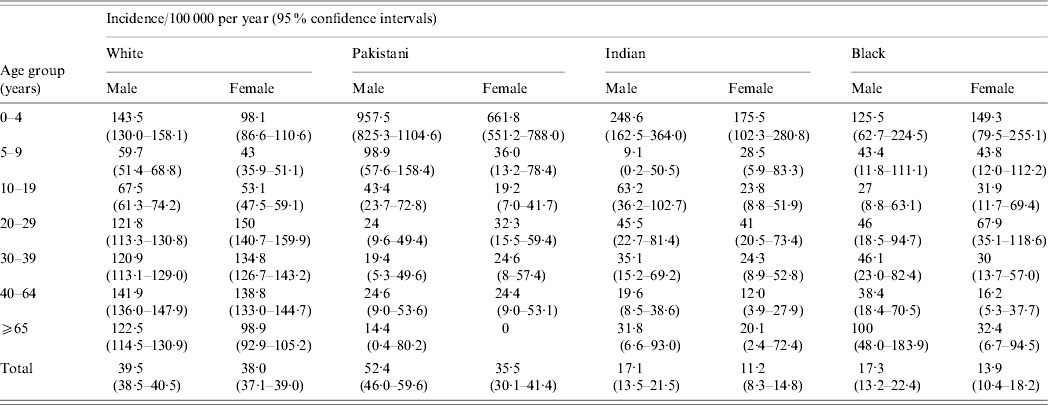
Patient age and gender was available for 12 309 of 12 327 cases (99·9%) in the ethic groups (White, Pakistani, Indian and Black) where numbers were sufficient for further analysis (Table 1). The incidence in male Pakistanis aged 0–4 years was higher than in female Pakistanis in this age group and than any of the other age/gender groups in the studied ethnic groups. In white males, the incidence was greater than in females at 0–4 years, 5–9 years and 10–19 years, but not at 20–29 years. In the resident Indian and Black populations no significant differences by age and gender were observed.
Table 1. The incidence by age and gender of indigenous Campylobacter infection in the main ethnic groups resident in the Campylobacter Sentinel Surveillance Scheme population (n=12 309), England and Wales, May 2000 to April 2003
Socioeconomic classification
A total of 3906 different occupational descriptions were provided by the 12 388 cases aged between 16 and 74 years, and these were classified into NS-SEC Analytical Class (‘AC’, Table 2). Overall incidence in white-collar workers (‘of or relating to work done in an office or other professional environment’ [26]) was marginally higher than in blue-collar workers (‘of or relating to manual work or workers’ [26]; RR 1·06, 95% CI 1·01–1·11, P=0·01), although incidence was highest in people working in semi-routine occupations (RR 1·73, 95% CI 1·64–1·81, P<0·001).
Table 2. The incidence by National Statistics – Socioeconomic Class (NS-SEC) of indigenous Campylobacter infection in the Campylobacter Sentinel Surveillance Scheme population (n=12 309), England and Wales, May 2000 to April 2003
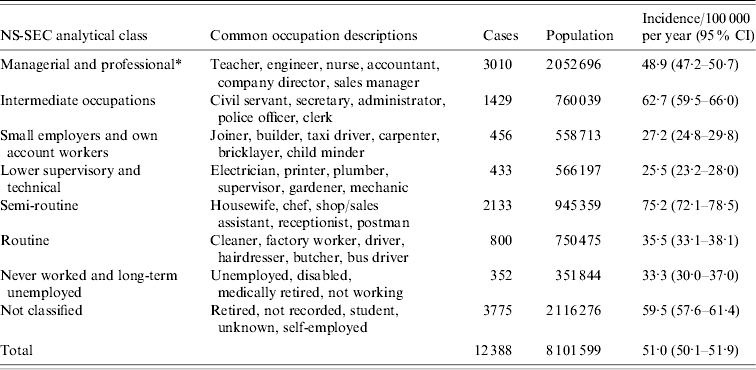
CI, Confidence interval.
* Higher managerial and professional occupations and lower managerial and professional occupations combined.
Age and gender were available for all 8261 cases in the main ACs (Fig. 2 a, b) and incidence differed greatly within and between genders. Although based on small numbers, the incidence in Small Employers and Own Account workers in both gender groups was higher than other ACs in the <20 years age group (RR 2·54, 95% CI 1·34–4·80, P=0·004), declined rapidly to 30–34 years (χ2 for trend 14·1, P<0·001) and more gradually further up the age spectrum (χ2 for trend 2·9, P=0·09). In Managerial and Professional (RR 1·17, 95% CI 1·05–1·31, P=0·01), Small Employers and Own Account (RR 1·79, 95% CI 1·16–2·76, P=0·02), Semi-Routine (RR 1·14, 95% CI 1·02–1·28, P=0·04) and Routine (RR 1·37, 95% CI 1·12–1·67, P=0·007) workers, the risk in females aged 20–24 years was significantly higher than for males in the same occupational groups. In Intermediate workers the risk of infection increased with increasing age up to 34 years (χ2 for trend 28·2, P<0·001) with no difference in risk between males and females (RR 0·98, 95% CI 0·87–1·11, P=0·72). The risk in male Intermediate workers then increased (χ2 for trend 5·08, P=0·02) to a peak in the 50–54 years age group, exceeding the risk in female Intermediate workers (RR 1·44, 95% CI 1·30–1·60, P<0·001) and other male workers in the 35–54 years age group (RR 2·13, 95% CI 1·88–2·40, P<0·001).
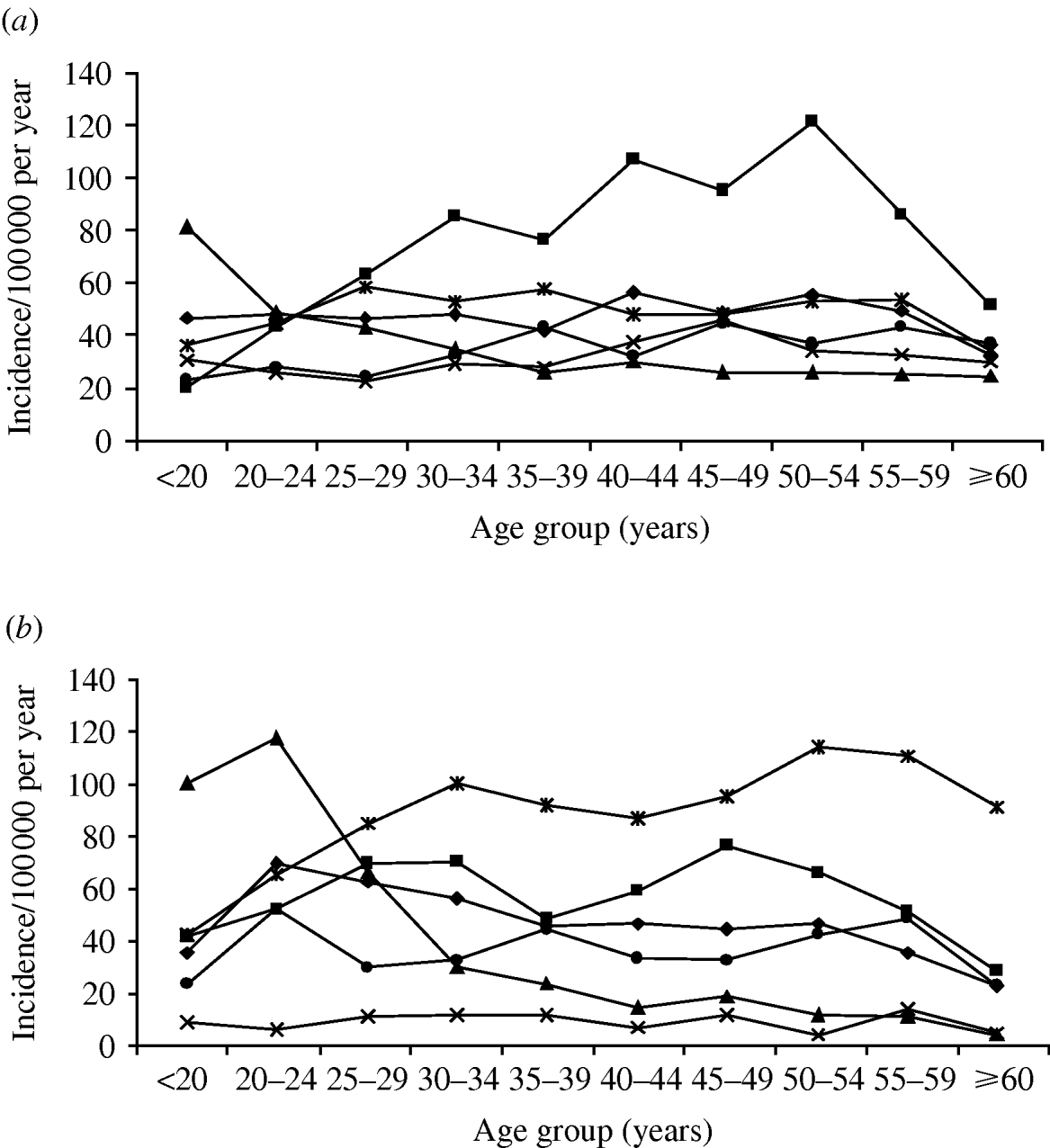
Fig. 2. The incidence by age group and National Statistics – Socioeconomic Class (NS-SEC) of indigenous Campylobacter jejuni infection in (a) males and (b) females in the Campylobacter Sentinel Surveillance Scheme population (n=8261), England and Wales, May 2000 to April 2003. –◆–, Managerial and Professional; –■–, Intermediate; –▲–, Small Employers and Own Account; –×–, Lower Supervisory and Technical – ![]() –, Semi-routine; –●–, Routine.
–, Semi-routine; –●–, Routine.
DISCUSSION
The comparison of comprehensive population-based surveillance data with detailed denominator data for the population from which the cases arose has enabled us to gain a valuable insight into the demographic characteristics of Campylobacter infection in England and Wales. The population covered by the Campylobacter Sentinel Surveillance Scheme has meant that the dataset generated is large and more geographically representative of the population of England and Wales. The coincidental timing of the study in terms of the United Kingdom 2001 Census has resulted in the availability of highly specific, accurate and relevant denominator data. Our study has re-emphasized that age and gender are major determinants for Campylobacter infection in England and Wales and has demonstrated that ethnicity, occupation and socioeconomic status are also important.
Where significant differences within and between demographic groups were identified, additional analyses of accompanying exposure data were undertaken to try to explain the increased risk. The prevalence of exposure was investigated, both between genders within age groups and between age groups within genders. Few differences were identified. This might relate to the fact that our exposure questionnaire is not exhaustive and covers a broad (14-day) exposure period, making differences difficult to detect, or that incidence differences relate to factors not connected with exposure. Our data did not allow us to disentangle these, since suitable control populations were unavailable.
The relationship between age and gender and the incidence of Campylobacter infection described in this study has not been described previously in such detail [Reference Skirrow27]. Infants and young children with infectious intestinal disease are more likely to present to primary-care physicians than older children and adults [28], therefore the observed increased incidence in <2-year-olds is not unusual. It is surprising, however, that within this age group the incidence in males was significantly higher than that in females and that this effect was noted each year from birth to 17 years. Few differences in exposure were noted between males and females in this age group, suggesting that other factors might have a role. The high male to female relative risk between 13 and 15 years is particularly intriguing. This period corresponds to the peak in puberty in males and it is possible that hormonal changes occurring at this time might affect the growth of Campylobacter present in the human gut. Hormones have been shown to have a positive effect on the growth of Campylobacter spp. in vitro, by enhancing their aerotolerance [Reference Bowdre29]. Recent research suggests that their presence might also increase pathogenicity [Reference Cogan30].
The increase in incidence in females from 14 years and the ‘switch’ in relative risk from males to females from 18 years to 36 years is also remarkable. This period corresponds to the main childbearing age in women, and accompanying hormonal (endogenous or exogenous) changes could affect women's susceptibility to infection. Both oestrogen and progesterone have been shown to positively affect the growth of C. rectus in vitro and this is thought to be an important factor in periodontal disease progression in pregnant women [Reference Yokoyama31]. Oral contraceptives will increase the concentrations of one or both of these hormones in the gut. Furthermore, the pattern of oral contraceptive use in the United Kingdom by age correlates well with the incidence of campylobacteriosis in women described in this study [Reference Taylor, Keyse and Bryant32]. Alternatively, female cases in this age group could represent co-primary or secondary infections if they are exposed at the same time as their children or subsequently infected by them. Furthermore, the age- and gender-specific effects described above may also relate to behavioural differences which exist between males and females in certain age groups (e.g. greater exposure to the outside environment in males due to football, rugby, etc.) not covered by our questionnaire. Additional work is required to explain the distinct risk profiles in men and women at different ages of life.
The increased incidence in the indigenous Pakistani community in England and Wales has already been described [33]. Previously we were unable to quantify the risk further, as age- and gender-specific denominator data were unavailable. Here we are able to confirm that infants and young children in all the main ethnic groups resident in England and Wales are at increased risk of infection compared with older people in these groups. However, the incidence in Pakistani infants and young children, and in males in particular, far exceeded that in the other main ethnic groups. Further study of this subset of the population is required to identify the causes of this increase.
The pattern of infection in the indigenous adult white population in England and Wales is in contrast to that observed in developed countries [Reference Coker34], where incidence is very high and low in childhood and adulthood respectively. Repeated exposure to multiple Campylobacter spp. at an early age in hyperendemic regions probably provides a high level of general immunity, whereas episodes of infection in developed countries arise mainly from single strains, providing little cross-immunity against other subtypes [Reference Skirrow, Hausler and Sussman35]. This does not, however, explain the lack of disease in indigenous adult Indians and Pakistanis in England and Wales in this study, unless immigration or previous travel to endemic regions has played a role.
Consideration of a number of methodological issues is required to contextualize the findings from this study and to inform on future studies of this kind. First, including all cases of Campylobacter infection in our study might have masked species-specific demographic factors, as previous research has demonstrated different risk exposures in cases of C. jejuni and C. coli infection [Reference Gillespie20]. However, typing data were available for only 63% of cases and therefore the specificity gained would have been at the expense of statistical power. An analysis of the C. jejuni subset, which gave similar findings to the ones described in this study and are available on request, confirm this. Furthermore, as 92% of laboratory-confirmed campylobacters in England and Wales are C. jejuni our findings are likely to relate more to this species than to others.
Second, providing patients with free text fields to describe their ethnic origin or occupation led to missing or unclassifiable responses, and increased the possibility of misclassification during coding, all of which might have affected our incidence estimates for some ethnic or socioeconomic groups, although this would be difficult to measure. Future studies of this kind should overcome this shortfall by providing categorical responses to the demographic questions posed. Similarly, the two-stage process of deriving socioeconomic status from patients' occupation descriptions could have led to errors in misclassification or transcription, and some occupational descriptions might have been wrongly assigned to NS-SEC by using the simplified derivation method. Classification and transcription was carried out by two contributors, however, and results were compared to minimize error, and the simplified technique for deriving NS-SEC still provides a high level (>83%) of agreement with the full method [24].
Finally, we were unable to control for all the factors under investigation in a single analysis, increasing the possibility of uncontrolled confounding. For example, it is possible (although unlikely, given the age distribution) that part of the observed risk in certain ethnic groups is mediated by their socioeconomic status and/or occupation. This potential drawback could have been overcome by applying multivariate regression techniques to the data, which would also have allowed for the effect of season to be examined. Such techniques require denominator data stratified by all factors under investigation, and these were unavailable. Given the developments in information technology over the last 20 years, national population data should be able available in a more dynamic form in the future.
In conclusion, age, gender, ethnicity and socioeconomic class are all important determinants of Campylobacter infection and epidemiological studies which fail to account for these effect modifiers, in design and/or analysis, might mask important risk factors for infection, if factors positively associated with disease in one demographic subset are protective in another. Future epidemiological studies on Campylobacter infection need to be of sufficient size to allow subgroup analyses within conceptual frameworks, or are focused on specific high-risk groups. With regard to the latter, there is a clear need to elucidate further the high observed disease incidence in Pakistani children resident in the United Kingdom.
APPENDIX
The Campylobacter Sentinel Surveillance Scheme Steering Committee
Mr A. Charlett (Head, Statistics, Modelling & Bioinformatics, Health Protection Agency Centre for Infections); Dr J. M. Cowden (Consultant Epidemiologist, Health Protection Scotland); Mrs J. A. Frost (Welsh Assembly, Cardiff, UK); Mr I. A. Gillespie (Clinical Scientist, Environmental and Enteric Diseases Department, Health Protection Agency Centre for Infections); Ms J. Millward (Principal Environmental Health Officer, Birmingham City Council); Dr K. R. Neal (Clinical Reader in the Epidemiology of Communicable Diseases, Division of Epidemiology and Public Health, University of Nottingham); Prof. S. J. O'Brien (Division of Medicine & Neuroscience, University of Manchester, UK); Dr M. J. Painter (Consultant in Communicable Disease Control, Manchester Health Authority); Prof. Q. Syed (Regional Director, Health Protection Agency North West); Dr D. Tompkins (Regional Microbiologist, Health Protection Agency Yorkshire & the Humber).
The Campylobacter Sentinel Surveillance Scheme Collaborators
Public health, environmental health and laboratory staff who served the populations of the following health authorities:
Birmingham, Bradford, Bro Taf, Bury & Rochdale, Dyfed Powys, East Kent, Barnet, Enfield & Haringey, Herefordshire, Leeds, Leicestershire, Manchester, North Cumbria, North Essex, North West Lancashire, Nottingham, Salford & Trafford, South & West Devon, South Lancashire, Southampton & South West Hampshire, Stockport, West Pennine, Wigan & Bolton.
In association with:
HPA Laboratory of Enteric Pathogens, Campylobacter Reference Unit; HPA Centre for Infections, Environmental and Enteric Diseases Department; HPA Local and Regional Services; HPA Statistics Unit.
DECLARATION OF INTEREST
None.






