INTRODUCTION
Toxoplasmosis is caused by an obligate intracellular protozoan Toxoplasma gondii. This parasite is able to infect various animal species as intermediate host, but only Felidae such as domestic cats shed oocysts. Intermediate hosts, e.g. cattle, sheep and pigs can be infected through ingestion of the oocysts. Humans become infected with T. gondii through ingestion of tissue cysts in undercooked meat from intermediate hosts, through ingestion of oocysts that have been shed into the environment by cats, and relatively less frequently through transplantation of an organ with a tissue cyst [Reference Tenter, Heckeroth and Weiss1, Reference Hill and Dubey2]. Human toxoplasmosis is usually subclinical or with non-specific symptoms like fatigue and general malaise. Clinical symptoms are lymphadenopathy and ocular disease, and toxoplasmosis can be fatal in immunocompromised patients [Reference Hill and Dubey2–Reference Gilbert4]. Primary infection during pregnancy may cause spontaneous abortion or stillbirth. An unborn child exposed to T. gondii in utero may develop congenital toxoplasmosis with major ocular and neurological consequences [Reference Hill and Dubey2, Reference Weiss and Dubey3, Reference Montoya and Liesenfeld5]. Due to its long-term complications and the fact that T. gondii is widely present in our environment, knowledge of the disease epidemiology and seroprevalence help shape health policies for prevention, particularly focused on pregnant women or women of childbearing age.
Seroprevalence rates vary between different European countries from 9% in the UK, 14–26% in Sweden (Stockholm 14%, South Sweden 26%), 28% in Denmark, up to 44% in France [Reference Berger6–Reference Evengård9]. Although differences between countries are partly due to different laboratory methods and study populations, there appears to be an increasing west–east and north–south gradient [Reference Pappas, Roussos and Falagas10]. In a prospective cohort study in The Netherlands that started in 1987 involving 28 000 pregnant women, seroprevalence at enrolment was 45·8%. This study, the Toxoplasma Intervention Prevention (TIP) study, was conducted in the Southwest of The Netherlands, essentially the city of Rotterdam and its surroundings [Reference Conyn-van Spaendonck11]. In 1995 and 1996, a population-based Toxoplasma seroprevalence study was performed in The Netherlands [Reference Kortbeek12]. The sera were obtained from a serum bank of the general population, aged 0–79 years, primarily designed for evaluation of the national immunization programme (NIP) [Reference De Melker and Conyn-van Spaendonck13, Reference De Melker, Nagelkerde and Conyn-van Spaendonck14]. This serum bank also offered the opportunity to obtain insight into other infectious diseases. Based on 7521 sera in 1995/1996, the overall seroprevalence of IgG antibodies against T. gondii was 40·5%, and 35·2% in women of reproductive age. Independent risk factors for acquired toxoplasmosis were: living in the Northwest; having occupational contact with animals; living in a moderately urbanized area; being divorced or widowed; born outside The Netherlands; frequent gardening and keeping a cat. Unfortunately, plausible risk factors such as consumption of raw or undercooked meat and vegetables could not be studied because no such information had been collected [Reference Kortbeek12]. Recently, a second population-based Toxoplasma seroprevalence study was performed with an adapted questionnaire in the general population of The Netherlands, using the serum bank which had been established to evaluate the NIP in 2006 and 2007. Our main objectives were to study the change in Toxoplasma seroprevalence in The Netherlands, and to determine current risk factors for acquired toxoplasmosis.
METHODS
Study population and questionnaire
To ensure maximum comparability, the study design for the second serum bank in 2006/2007 was kept similar to that of the first serum bank in 1995/1996. The study design and details on the data collection of these cross-sectional population-based studies have been published elsewhere [Reference De Melker and Conyn-van Spaendonck13–Reference van der Klis15]. In short, to establish a serum bank of the general population in The Netherlands, eight municipalities were sampled within each of five geographical Dutch regions, and eight additional municipalities with low immunization coverage were sampled, resulting in 48 municipalities (see Fig. 1). Nine municipalities that were sampled in 1995/1996 were sampled again in 2006/2007. An age-stratified sample (age groups <1, 1–4, 5–9, …, 75–79 years) was randomly taken from each municipality. In total 17 341 persons were invited to participate in the national sample and 4376 in the sample with low immunization coverage municipalities. In 12 of these 40 municipalities an oversampling of non-Western migrants was carried out. In total 2574 migrants were invited to participate. Subjects were requested to give a blood sample and to complete a questionnaire. The questionnaire inquired about demographic characteristics, vaccination history, health perception and diseases, activities possibly related to infectious diseases (e.g. travelling, profession, food habits, gardening), and information related to sexually transmittable diseases for 15- to 79-year-olds. Samples and data of the currently described study were collected in the period from February 2006 to June 2007.

Fig. 1. Map of the 48 municipalities sampled to establish a national serum bank of the general population in The Netherlands in 1995/1996 and 2006/2007.
Antibody assay
The sera were stored at −80°C. Antibodies against T. gondii were determined in a sandwich ELISA with a serum dilution of 1:20 (adapted from a previously described method [Reference Ruitenberg and van Knapen16]). The antigen is derived from a crude extract of a Toxoplasma RH strain, the conjugate is a peroxidase-labelled anti-human IgG conjugate (Dako, Denmark). A cut-off serum was used and its optical density value was allowed to vary between 0·10 and 0·30. The methods, antigens and controls have not altered over the past 25 years. Therefore, the results of the first and second Toxoplasma seroprevalence study are comparable. The extinction value of the tested serum and the cut-off serum was used to calculate a ratio. A ratio of <1 was considered to be negative; a ratio of at least 1·0 to be positive. Sufficient serum was available for 7030/7904 participants. The missing participants consisted mostly of children aged <1 year because a smaller amount of serum was collected from these children, which had already been used for evaluation of diseases in the NIP.
Statistical analysis
To determine the seroprevalence of T. gondii IgG antibodies representative of the general population of The Netherlands, all 1489 participants from low immunization coverage municipalities were excluded from the seroprevalence estimation. The migrant participants were included in the seroprevalence estimation. Therefore, seroprevalence was weighted within each municipality for age and gender, and also for ethnicity and urbanization degree, up to their proportion in the total population of The Netherlands (on 1 January 2007). In the seroprevalence analysis, we also adjusted for the two-stage cluster sampling by taking into account the strata (five regions) and clusters (40 municipalities). Except for the uncorrected prevalence rates in Table 2, all seroprevalence estimates in this paper are weighted. Based on the number of pregnant women per age group and the yearly change of the weighted seroprevalence of T. gondii IgG antibodies in the general population, the incidence of live-born children with congenital toxoplasmosis could be estimated for the 1995/1996 and 2006/2007 surveys. Prevalence rate per age group and yearly change were estimated using spline functions [Reference Ramsay17]. The uncertainty in the congenital toxoplasmosis estimates was based on recalculation of 10 000 bootstrap samples from the two surveys. During pregnancy we assumed a median probability of mother-to-child transmission of one third [Reference Gilbert and Gras18, Reference Dunn19].
The participation rate was 55% in the nationwide sample of 1995/1996 and 33% in 2006/2007. Socio-demographic data are available from non-responders, and have been published elsewhere [Reference van der Klis15]. Similar to the analysis in 1996, logistic regression analysis was used to determine whether any of the following variables were independent predictors of seropositivity for T. gondii, after adjustment for age group and gender. In contrast to the weighted seroprevalence estimation, all 7030 participants were included in the logistic regression to increase power, as living in a low immunization coverage municipality was not significantly associated with Toxoplasma seropositivity. A model was developed using multivariate logistic regression with backwards elimination. Variables which reached a significance level of P⩽0·10 in the univariate analyses were selected for inclusion in the multivariate logistic regression model. The selected variables for multivariate logistic regression were: geographical region; degree of urbanization; country of birth; religion; educational level; household income; number of persons per household; consumption of raw pork, raw beef or raw mutton; consumption of raw unwashed vegetables; vegetarianism; gardening; putting sand in mouth while playing in sandbox (only for children aged <15 years); keeping a cat, bird or dog in the past 5 years; keeping cattle, sheep, pigs or goats in the past 5 years; tick bites in the past 5 years; sun allergy; hay fever; eczema; bronchitis; and having occupational contact with clients or patients in the past 5 years.
After development of a model with the smallest number of statistically significant (P⩽0·05) variables for the 2006/2007 survey through backwards elimination, some non-significant variables were added to the multivariate model to allow for comparison with the multivariate model derived in the previous population-based Toxoplasma seroprevalence study [Reference Kortbeek12]. These additional non-significant variables were: marital status, country of birth, keeping a dog or a rabbit, hamster or guinea pig in the past 5 years, gardening, and having occupational contact with animals in the past 5 years. Separate models were developed for the 0–19 and ⩾20 years age groups. Statistical analyses were performed with SAS v. 9.1 (SAS Inc., USA).
RESULTS
Prevalence of T. gondii IgG antibodies
Out of the nationwide sample of 7030 sera including participants from low immunization coverage municipalities, 1806 (26·4%) tested positive for IgG antibodies against T. gondii. Excluding participants from low immunization coverage municipalities to enhance representability for the general population, 1423/5541 sera tested positive for IgG antibodies against T. gondii. The overall weighted estimate of Toxoplasma seroprevalence for the general population of The Netherlands, decreased markedly from 40·5% (95% confidence interval 37·5–43·4) in 1995/1996 to 26·0% (95% CI 24·0–28·0) in 2006/2007 (see Table 1). Similar to 1995/1996, there were clear geographical differences, with the highest seroprevalence in the western part of the country. No differences were found between men (25·9%, 95% CI 23·2–28·5) and women (26·1%, 95% CI 24·0–28·2). The average seroprevalence for women of reproductive age (15–49 years) was 18·5% (95% CI 16·2–20·7), leaving the majority of pregnant women susceptible to primary infection with T. gondii. The current seroprevalence is significantly lower than in 1995/1996 when the seroprevalence in this group was 35·2% (95% CI 32·9–38·6), and in 1987 when the seroprevalence was 45·8% (95% CI 45·2–46·3) in the TIP study. In general, seroprevalence rose with age (Fig. 2), with the steepest slope in the reproductive age group of 15–49 years. Based on the number of pregnant women per age group and the yearly change of the weighted seroprevalence in the general population, aged 15–49 years, the incidence of live-born children with congenital toxoplasmosis could be estimated. The estimated incidence of congenital toxoplasmosis decreased significantly (P=0·0037) from 3·1/1000 (95% CI 2·6–3·6) live-born children in 1996, to 2·2/1000 (95% CI 1·7–2·7) live-born children in 2007.

Fig. 2. Age-specific prevalence of Toxoplasma gondii IgG antibodies in the first national serum bank in 1995/1996 (– – –; n=7521) [Reference Kortbeek12], and in the second national serum bank in 2006/2007 (——; n=5541). Prevalence rate per age group were estimated using spline functions.
Table 1. PrevalenceFootnote * of Toxoplasma gondii antibodies in the first national serum bank in 1995/1996 and in the second national serum bank in 2006/2007, stratified for gender, degree of urbanization, being born in The Netherlands, and ethnicity
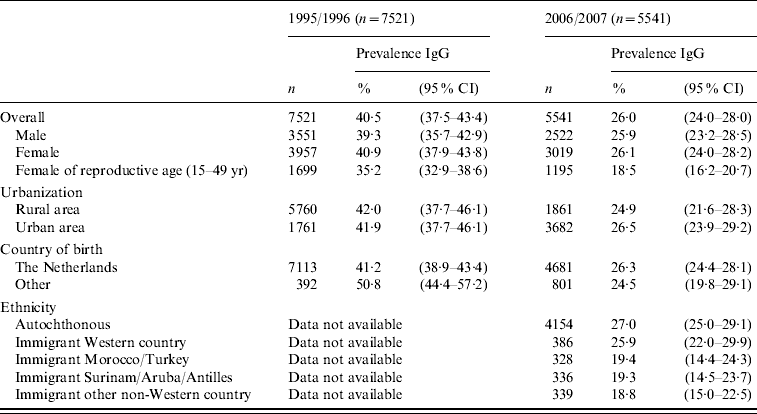
CI, Confidence interval.
* Weighted prevalence for age and gender, ethnicity and urbanization degree.
Predictors of seropositivity for T. gondii IgG antibodies
Only statistically significant predictors of Toxoplasma seropositivity are shown in Table 2. For participants aged 0–19 years, the only significant risk factor associated with Toxoplasma seropositivity was keeping cattle or sheep in the past 5 years. Two additional independent risk factors were statistically significant when the logistic regression analyses with young participants was restricted to the 0–15 years age group: consumption of raw unwashed vegetables [adjusted odds ratio (aOR) 1·5, 95% CI 1·0–2·1] and putting sand in mouth while playing in sandbox (aOR 1·6, 95% CI 1·0–2·6).
Table 2. Uncorrected prevalence of specific antibodies to Toxoplasma gondii (%) and multivariate logistic regression analyses of risk factors associated with seropositivity in participants aged 0–19 and 20–79 years
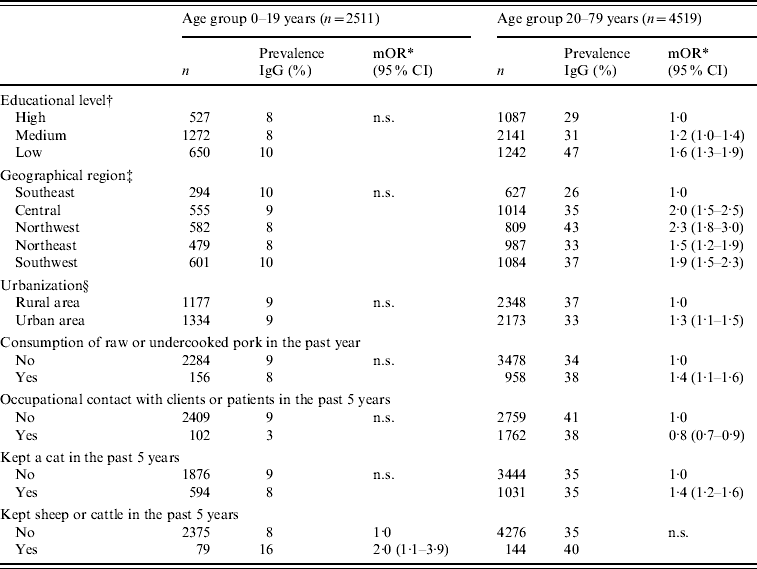
mOR, Multivariate odds ratio; CI, confidence interval; n.s., not significant.
* Adjusted for age and gender.
† The following categories were used for educational level of those aged ⩾15 years and of one the parents for those aged <15 years: ‘low’ (primary school, lower vocational or lower general secondary education); ‘medium’ (intermediate vocational or intermediate general secondary and higher general secondary education); ‘high’ (higher vocational secondary education and university education).
‡ The geographical regions were based on the Dutch provinces: ‘Central’ (Utrecht, Gelderland); ‘Southeast’ (Noord-Brabant, Limburg); ‘Northwest’ (Noord-Holland, Flevoland); ‘Southwest’ (Zeeland, Zuid-Holland); ‘Northeast’ (Groningen, Drenthe, Overijssel, Friesland).
§ The following categories were used for level of urbanization: ‘urban’ (>1500 addresses/km2) and ‘rural’ (<1500 addresses/km2).
For participants aged 20–79 years, Toxoplasma seropositivity was independently associated with low educational level, living in an urban area, consumption of raw or undercooked pork, keeping a cat, and living in the Southwest, Central, Northwest or Northeast region of The Netherlands, compared to the Southeast. Occupational contact with clients or patients in the past 5 years was negatively associated with Toxoplasma seropositivity.
DISCUSSION
This study showed a decreased seroprevalence of toxoplasmosis from 40·5% to 26·0% during the past decade. We observed an even stronger decrease in women of reproductive age, from 35·2% in 1995/1996 to 18·5% in 2006/2007. A decline in seroprevalence has also been reported in other developed countries [Reference Pappas, Roussos and Falagas10], and has been attributed to the introduction of modern farming systems resulting in a lower prevalence of Toxoplasma cysts in meat, in combination with an increased use of frozen meat by consumers [Reference Tenter, Heckeroth and Weiss1, Reference Kijlstra and Jongert20, Reference Dubey and Jones21]. Data on seroprevalence of toxoplasmosis in Europe are limited. The European Toxo Prevention Study Group (EUROTOXO) performed a survey in 2004 on surveillance systems, data on seroprevalence and risk factors studies in Europe. They reported that two countries have a surveillance of congenital toxoplasmosis on a national basis, France and Germany (screening and surveillance in Denmark were stopped in July 2007 [Reference Bénard22]). In a systematic review only few articles on seroprevalence of toxoplasmosis in the general population were defined as representative [Reference Bénard, Salmi and Mouillet23]. Risk factors for Toxoplasma infection vary according to local food customs, food hygiene and lifestyles in different countries [Reference Cook24–Reference Kortbeek26]. Our data show a continued decreasing trend in accord with the previous study by Kortbeek and colleagues when seroprevalence had decreased significantly compared to the TIP study in 1987 [Reference Conyn-van Spaendonck11, Reference Kortbeek12]. The estimated incidence of live-born children with congenital toxoplasmosis also decreased from 3·1 to 2·2/1000 live-born children between 1996 and 2007, but is still substantial. This estimate is consistent with our recent study on the incidence of congenital toxoplasmosis in The Netherlands. Based on T. gondii-specific IgM antibodies in dried-blood spot filter paper cards from 10 008 newborns, the incidence of congenital toxoplasmosis in The Netherlands was estimated at 2·0 (95% CI 1·3–3·0) per 1000 live-born children in 2006, which is relatively high compared to other European countries [Reference Kortbeek26].
By multivariate logistic regression we found that living in the Northwest of The Netherlands, living in an urban area, low educational level, consumption of raw or undercooked pork, keeping a cat, and having occupational contact with clients or patients were independently associated with Toxoplasma seropositivity in participants aged ⩾20 years. However, the odds ratios were small and some risk factors that were identified in 1995/1996 were no longer statistically significant in 2006/2007. For participants aged 0–19 years, country of birth, gardening, degree of urbanization, and geographical region were no longer independently associated with Toxoplasma seropositivity. For participants aged 20–79 years, marital status, gardening, having occupational contact with animals, and keeping a rabbit were no longer independently associated with Toxoplasma seropositivity [Reference Kortbeek12]. This is probably partially due to the lower Toxoplasma seroprevalence in 2006/2007.
We have no explanation yet for the geographical differences or for the effect of urbanization. Similar geographical differences were seen in 1995/1996, with the highest risk in the Northwest and lowest in the Southeast for adults. For participants aged <20 years the geographical differences appear to have declined and are no longer independently associated with Toxoplasma seropositivity. Moreover, interpretation is difficult because only residence at the moment of our study was known and not the places of birth and childhood for participants born in The Netherlands. The geographical differences could reflect regional differences in demographic characteristics, such as ethnicity or religious habits, or differences in consumption of specific regional food items in the different parts of The Netherlands. However, neither ethnicity nor religious habits were independent predictors of Toxoplasma seropositivity. Although the questionnaire inquired about consumption of raw or undercooked meat during the preceding year, it was not designed to record precise consumption habits. Apart from type of meat (e.g. pork, beef, mutton, poultry), no inquiry was made for specific products, so high-risk regional food items such as regional raw meat sausages could remain unnoticed, and are not accounted for in the analyses. Low educational level was an independent predictor for Toxoplasma seropositivity in the 20–79 years age group, and the risk was lower in participants who reported occupational contact with clients or patients. This might be a result of differences in food habits and hygiene standards.
Inadequately cooked or raw meat is widely acknowledged as a main risk factor for infection with Toxoplasma. Several types of meat are likely to be involved in the transmission of tissue cysts, and have been identified as a risk factor, e.g. beef, mutton, pork and game meat [Reference Hill and Dubey2, Reference Cook24, Reference Kapperud27]. In our study, consumption of mutton and game meat were reported rarely, respectively by ten (0·14%) and four (0·06%) out of 7030 participants. Although consumption of raw beef was reported by 50% of the participants, this type of meat was not identified as risk factor. However, we did identify consumption of inadequately cooked or raw pork as an independent predictor of Toxoplasma seropositivity. In developed countries, including The Netherlands, the rate of T. gondii infection of pork has dropped dramatically due to major changes in animal production hygiene [Reference Tenter, Heckeroth and Weiss1, Reference Dubey and Jones21]. Currently, modern production systems have virtually eliminated Toxoplasma infection in pigs. However, animal-friendly production systems with a higher risk of exposure to Toxoplasma are increasing in popularity, and may cause a re-emergence of pork as an infectious meat source [Reference Kijlstra and Jongert20]; in 2004, Toxoplasma seroprevalence in pigs from intensive farming systems in The Netherlands was virtually nil (0·4%), but 5·6% in free ranging pigs [Reference van der Giessen28].
Similar to our multivariate model in 1995/1996, keeping a cat in the past 5 years remained an independent predictor of Toxoplasma seropositivity. The questionnaire also inquired about contact with cats in the past year, e.g. stroking or playing with them, and whether these were kittens or cats aged >1 year. Replacement of the variable for cat ownership by the variable for contact with cats (reported by 51% of participants) yielded almost identical odds ratios in the multivariate logistic regression model. Contact with kittens, which was reported by 13% of the participants, was not an independent risk factor, in contrast with a recent study in the USA [Reference Jones29]. Raising kittens would be a plausible risk factor, because they are often exposed to T. gondii as soon as they develop hunting skills and have access to rodents and birds or are exposed to oocysts in soil. The kitten would shed oocysts 1 or 2 weeks after primary infection, and after that cats are considered to be immune to reshedding of oocysts, except after superinfection with other coccidia or after immunosuppression under experimental conditions [Reference Tenter, Heckeroth and Weiss1, Reference Dubey30, Reference Davis and Dubey31]. However, in our study we did not find an increased risk associated with contact with kittens, although we did find an association with cats aged >1 year. This might be due to misclassification of the cat's age by participants. Possibly we should have enquired about raising a litter of kittens, as in the study by Jones et al. [Reference Jones29] the increased risk associated with exposure to kittens was limited to respondents who had ⩾3 kittens, suggesting that raising a litter of kittens may be responsible for the risk.
For the younger participants, the only risk factors were keeping sheep or cattle, consumption of raw unwashed vegetables, and putting sand from a sandbox in the mouth. Growing up on a farm as a risk factor was reported by a study from the UK [Reference Nash7]. Further analysis of our data suggested that keeping a cat may contribute to the higher risk of T. gondii infection in children who grow up in a home that also keeps cattle or sheep, as cats are reported more often by owners of cattle and sheep.
Although it is generally assumed that raw vegetables are a source of contamination with T. gondii in humans through contamination with soil containing oocysts, to date sparse experimental data or studies are available to support this route of infection. One study has shown that berries experimentally spiked with oocysts can pass T. gondii infection to mice, and two case-control studies report consumption of raw vegetables as a risk factor in pregnant women [Reference Kapperud27, Reference Kniel32, Reference Baril33]. Another age-specific risk factor related to childhood was putting sand in the mouth while playing in a sandbox. Sandboxes have been recognized as a source of infection for several pathogenic viruses, bacteria and parasites [Reference Matsuo and Nakashio34–Reference Werber36], but T. gondii oocysts have only recently been isolated from sandboxes [Reference Lass37]. The presence of oocysts in sandboxes creates a risk of contracting primary toxoplasmosis by children and even adults, which indicates the need for better protection of sandboxes from faecal contamination. This can be achieved by taking measures like systematic changing of the sand and/or covering the sandbox when not in use.
CONCLUSION
Along with the overall seroprevalence, the seroprevalence in women of reproductive age (15–49 years) in The Netherlands decreased from 35·2% in 1995/1996 to 18·5% in 2006/2007, leaving the majority of pregnant women susceptible to primary infection with T. gondii. Therefore, education about dietary and environmental sources of Toxoplasma infection remains essential to prevent toxoplasmosis and a range of other infections. Considering the fact that acquired toxoplasmosis can cause eye disease at all ages, and can be fatal to immunocompromised patients, this education may be as relevant to the general public as for pregnant women. For persons aged >20 years, the outcomes of risk-factor analyses did not indicate predictors of Toxoplasma seropositivity that not are already incorporated into the education programme for pregnant women. However, additional attention could focus on prevention of acquired toxoplasmosis in children through sandboxes and through consumption of raw vegetables.
DECLARATION OF INTEREST
None.






