INTRODUCTION
West Nile virus (WNV) is the leading cause of arthropod-borne viral encephalitis in the USA [Reference Reimann1]. Although the majority of persons infected with WNV remain asymptomatic, 20% develop a non-specific febrile illness and <1% develop neuroinvasive disease, which typically manifests as meningitis, encephalitis, or acute flaccid paralysis [Reference Mostashari2, Reference Sejvar and Marfin3].
Detection and reporting of WNV neuroinvasive disease cases is assumed to be more consistent and complete than non-neuroinvasive disease cases and is often used to estimate total burden of infection [Reference Lindsey4]. Reported WNV cases help guide public health education and control activities, and impact regional WNV blood product screening procedures [Reference Custer5]. Confirmation of WNV infections can also help healthcare providers by informing clinical management and prognosis.
Although prior studies have described WNV underreporting [Reference Boehmer6, Reference Silk7], none have investigated the completeness of WNV testing in patients with a clinically compatible illness. We reviewed cases of meningitis and encephalitis occurring during an outbreak of WNV in the East Valley of the Phoenix metropolitan area and assessed the proportion tested for WNV infection.
METHODS
We defined a case of meningitis or encephalitis as an East Valley resident admitted to an inpatient ward or seen in an emergency department (ED) with acute onset of fever and either neurological dysfunction or cerebrospinal fluid (CSF) pleocytosis (>5 leukocytes/mm3) during 1 May–31 August 2010. For the purpose of this study, the East Valley of metropolitan Phoenix was defined as the cities and towns of Apache Junction, Chandler, Gilbert, Mesa, Queen Creek, Tempe, Phoenix (zip code 85044 only), San Tan Valley (excluding zip code 85132), and other unincorporated areas occurring within the geographical boundaries of these towns and cities. A case of WNV neuroinvasive disease was defined as a case of meningitis or encephalitis with laboratory evidence of acute WNV infection, including anti-WNV immunoglobulin M (IgM) antibodies or WNV RNA detected in serum or CSF.
Meningitis and encephalitis cases were identified from patients who had CSF collected at six hospitals serving the East Valley (Fig. 1). Hospitals included in the study were larger institutions with high intake capacity where physicians were more likely to see and admit patients with neuroinvasive disease, and from which WNV cases had previously been reported. Four hospitals were situated in the East Valley and two were situated outside but adjacent to the East Valley. Each of the hospitals' laboratories compiled a list of all patients who had CSF collected over the specified time period, including demographics, date of admission, and date of CSF collection. We reviewed the list and excluded patients if they were aged <2 months (possible neonatal infections), had CSF collected ⩾4 days after admission (possible nosocominal infections), or lived outside the East Valley. We then randomly sampled one-third of the eligible patients and, based on a limited record review, excluded those who did not have infectious disease testing performed on CSF [i.e. bacterial culture, herpes simplex virus polymerase chain reaction (PCR), enterovirus PCR, or WNV serology]. Excluded patients were replaced with additional patients from the same hospital who met the above-mentioned criteria, if available. Finally, a complete medical record review was performed to identify patients who met the case definition for meningitis or encephalitis and lacked a known aetiology for their illness, other than WNV.

Fig. 1. Identification and sampling of meningitis and encephalitis cases – East Valley of metropolitan Phoenix, Arizona, 2010. * East Valley residents aged ⩾2 months admitted to an inpatient ward or seen in an emergency department with CSF collected ⩽4 days after admission. † Excluded patients with no infectious disease testing performed on CSF. ‡ The number of eligible patients from one facility was too small to replace all excluded patients.
For the identified meningitis and encephalitis cases, we collected data on patient demographics, clinical signs and symptoms, illness severity and outcomes, and laboratory testing results for potential infectious aetiologies, including WNV. Data were analysed using SAS version 9.2 software (SAS Institute Inc., USA) and Epi Info version 3.5.1 (CDC, USA). We performed χ2 or Fisher's exact tests for categorical variables and Wilcoxon's two-sample test for continuous variables.
RESULTS
We identified 60 patients with meningitis or encephalitis who met all inclusion criteria. Of these, 24 (40%) had WNV laboratory testing performed. The proportion of case-patients tested vs. those not tested did not differ by sex but testing increased significantly with increasing age (Table 1, Fig. 2). The median age of patients tested for WNV infection was 50 years (range 16–89 years) compared to 28 years (range 0·2–70 years) for those not tested (P<0·01). Only 12 (28%) of 43 patients aged <50 years were tested for WNV compared to 12 (71%) of 17 patients aged ⩾50 years (P<0·01). The proportion tested did not differ by hospital or city of residence, nor did it vary significantly over the time period evaluated. Testing for WNV was not associated with meningism, mental status changes, or pleocytosis; however, case-patients with weakness/paralysis and those with elevated CSF protein (>50 mg/dl) were more likely to be tested. Case-patients tested for WNV were more likely to have been admitted to the inpatient ward as opposed to being seen only in the ED (P=0·04) and, if admitted, to be discharged to a rehabilitation facility (P<0·01).

Fig. 2. Proportion of meningitis or encephalitis cases tested for West Nile virus (WNV), by age group – East Valley of metropolitan Phoenix, Arizona, 2010.
Table 1. Characteristics of meningitis and encephalitis cases by West Nile virus (WNV) testing status – East Valley of metropolitan Phoenix, Arizona, 2010
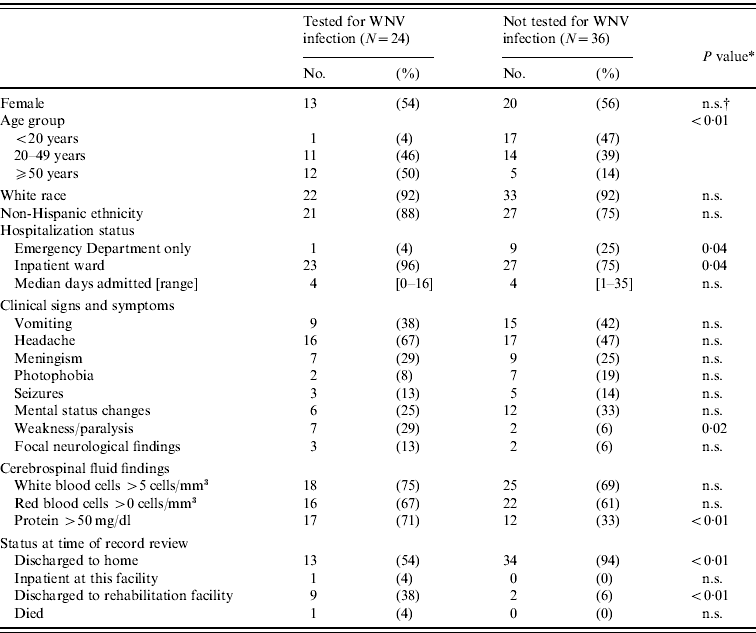
* χ2 test or Fisher's exact test for categorical variables.
† n.s., Not significant (P⩾0·05).
Of the 24 meningitis and encephalitis case-patients tested, five (21%) had laboratory-confirmed WNV neuroinvasive disease. Of the WNV-confirmed cases, three (60%) patients had meningitis and two (40%) had encephalitis. Two (40%) patients had a history of cancer, one of whom was under treatment at the time of diagnosis with WNV infection. Patients with WNV neuroinvasive disease were older (median age 72, range 50–89 years) than patients with meningitis or encephalitis who tested negative for WNV (median age 44, range 16–80 years) (P=0·02); no other significant differences were identified.
DISCUSSION
This is the first published report to estimate the completeness of WNV testing in patients with unexplained encephalitis or meningitis. Overall, we found that only 40% of patients presenting with a clinically compatible neuroinvasive illness were tested for WNV infection. Although the proportion tested improved with increased patient age and disease severity, the lack of testing in children, young adults, and those with less severe disease probably resulted in substantial underestimates of the WNV neuroinvasive disease burden. Furthermore, this study was conducted during a WNV outbreak with increased health department alerts and media coverage and we would expect the proportion of cases tested for WNV to be even lower in non-outbreak settings.
Clinicians may omit WNV testing for several reasons. Our data suggest that patient age, disease severity, clinical signs (weakness or paralysis), and CSF parameters were significant factors. Less than 30% of the meningitis and encephalitis patients aged <50 years were tested for WNV compared to >70% of patients aged ⩾50 years. Although the incidence of WNV neuroinvasive disease increases significantly with age, patients aged <50 years account for 55% of all WNV neuroinvasive disease cases nationally [Reference Lindsey4]. Of children aged <20 years, only one (6%) of 18 patients was tested for WNV. Even though cases of WNV neuroinvasive disease in children comprise only 5% of total cases reported nationally [Reference Lindsey4], the lack of testing means that the diagnosis is likely to be missed in this age group. Patients seen only in the ED were less likely to be tested for WNV, possibly due to their less severe presentation. However, this would not preclude the diagnosis, since up to 19% of patients with WNV neuroinvasive disease are not admitted to hospital [Reference Patnaik, Harmon and Vogt8–Reference Jean10]. Additionally, clinicians may question the impact of testing on management because of anticipated delays in receiving results and the absence of proven treatment options. Despite these shortcomings, diagnostic confirmation does inform clinical management (e.g. removing antibiotic therapy or testing for alternative aetiologies) and expectations regarding the patient's prognosis [Reference Sejvar11].
Our study was subject to several limitations. We conducted our review in larger hospitals servicing the area; testing practices in these facilities may have differed from other smaller facilities. By excluding patients who had no other infectious disease testing performed, we may have biased the sample towards those more likely to have had testing for possible WNV infection. Our case definition limited our ability to evaluate testing practices in WNV cases with less common or atypical presentations; for instance across the six institutions, we were aware of two patients who had CSF serological evidence of WNV infection but did not present with fever so they were not included in the analysis. Finally, we were unable to quantify the true rate of underdiagnosis because clinical specimens were no longer available for additional WNV testing to identify missed cases.
Detection of WNV in mosquitoes collected by environmental sampling can provide early warning of local WNV activity [Reference Unlu12]. However, ecological surveillance activities are now curtailed in many jurisdictions due to budgetary constraints, and public health and blood collection agencies are increasingly relying on human WNV case data to make decisions on prevention measures (e.g. targeting mosquito-control efforts or intensifying blood donor screening) [Reference Stramer13]. Providing better estimates of disease burden and location of human disease cases are critical to prevention efforts. Therefore, although human surveillance is not ideally suited for early regional detection of WNV activity [14], it should be optimized for use in combination with available environmental surveillance to aid in timely prevention measures. Based on our findings, we recommend that public health officials encourage healthcare practitioners to expand testing for WNV in persons with clinically compatible illness to include younger patients and those with less severe clinical disease. The true proportion of missed cases could be assessed prospectively by testing CSF samples submitted for other infectious disease testing for WNV to strengthen disease estimates for public health planning and response.
ACKNOWLEDGEMENTS
We gratefully acknowledge the infection control practitioners and medical records managers of the six facilities for kindly accommodating and assisting us during data collection and Mark Delorey and Kallie Horiuchi, Centers for Disease Control and Prevention, Fort Collins, CO for their input on sampling and analysis considerations.
The opinions expressed by authors contributing to this work do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
DECLARATION OF INTEREST
None.





