INTRODUCTION
In order to effectively control infectious diseases, surveillance is needed at different levels. Monitoring reported cases becomes an insufficient means of surveillance when vaccination programmes have been widely implemented and successfully lowered the incidence of the targeted diseases, especially when these are considerably underreported. Moreover, vaccination is known to change the epidemiology of the targeted diseases by slowing down their epidemic cycles and shifting upwards the age at infection. At this stage, information about remaining and potentially accumulating susceptibility within the population is highly important to adjust preventive policy. Serum surveys provide a reliable source of data to estimate the current risk of infection across age groups, and to predict evolutions in the future [Reference Osborne1–Reference de Melker and Conyn-van Spaendonck4].
In Belgium, 1 year after the first free universal vaccination campaign against poliomyelitis had been launched (in 1958), additional infant vaccinations against diphtheria (D), tetanus (T) and pertussis were implemented, also free of charge. A DT booster was added at age 6 years in 1964, and a T booster at 15–16 years in 1985, which was later (in 1995) replaced by a reduced D-antigen content (dT) booster. Also in 1985, universal measles-mumps-rubella (MMR) vaccination was launched with a first dose in the second year of life. A second MMR dose at 10–13 years was added in 1995, and replaced the monovalent rubella vaccination for pre-adolescent girls which had been widely administered since 1973. Hepatitis B (HBV), conjugate H. influenzae type b (Hib), meningococcal C and 7-valent pneumococcal vaccines have been incorporated into the schedule more recently. To cope with the rising number of antigens in the infant schedule, new combination vaccines have been included in the free-of-charge supply soon after becoming available. DTPa-IPV extemporaneously mixed with Hib was used from 2001 up to 2004, when the hexavalent DTPa-IPV-Hib-HBV vaccine was introduced.
Reported incidence rates of all targeted diseases dramatically declined soon after implementation of universal vaccination, indicating satisfactory uptake. Nevertheless, regional coverage surveys organized since 1989 in Wallonia (representing about 30% of the population), since 1999 in Flanders (about 60% of the population), and since 2000 in Brussels' capital region demonstrated suboptimal uptake of infant MMR at least up to 2000, and suboptimal uptake of all vaccinations recommended later in childhood (B. Swennen, personal communication) [Reference Vandermeulen5–Reference Theeten9]. Data about dT coverage in adults are scarce. A serological survey in 1993–1994 found high diphtheria susceptibility rates in adults before the implementation of adult dT [Reference Mathei10]. More recently, in 2002, participation in the European Seroepidemiology Network 2 (EU project ESEN 2) demonstrated relatively high measles and rubella seronegativity rates in Belgian children and young adults compared to other European countries [Reference Andrews11, Reference Nardone12].
To evaluate the current age-related susceptibility within the Belgian population, a new survey was performed in 2006. Here we report the results for measles, mumps, rubella, diphtheria, and tetanus.
METHODS
Study population
Residual samples were collected using a multi-tiered approach to reach a sufficient number of serum samples. Samples from children aged from 1 to 19 years were prospectively collected by diagnostic laboratories whereas samples from adults aged 20–65 years were retrieved from voluntary blood donors and collected by the blood transfusion centres (BTC) of the Walloon and the Flemish branch of the Belgian Red Cross. Only people living in Belgium were included. To obtain a geographically well distributed sample, 15 diagnostic laboratories were involved that were spread over the country's 10 provinces. They were allocated fixed numbers of samples per age group to enable collection proportionally to the population of each region (Flanders, Wallonia, Brussels capital region) in the first place, and to the population of each province in the second. The number of samples was further stratified by age and gender. In each age group equal numbers of males and females were aimed for. Age groups were defined per single age year up to age 19 years, and in larger age groups (5–10 years) for adult samples. Samples were collected from January to December 2006 in all participating laboratories, but some experienced logistic problems and were allowed to extend their collection up to October 2007. The target total number of samples (n=4170), and the above stratifications were based on the expected proportions per age group as well as previous experience with various age-specific analyses of seroprevalence data [Reference Osborne, Weinburg and Miller2, Reference Edmunds13].
To avoid selection of immunosuppressed subjects by using residual samples, specific selection criteria were communicated to the hospital laboratories. Samples preferably had to be collected from emergency, otorhinolaryngology, and surgery/orthopaedic wards. Samples from oncology or intensive care wards as well as samples for which information about an immunosuppressed condition or multiple transfusions was available were excluded. It should be noted that adults are routinely excluded from blood donation if they are immunosuppressed or received multiple transfusions.
For each sample, the birth date, sample date, gender and postal code of the place of residence were provided by the collecting laboratories. The region (Brussels, Flanders, Wallonia) and province of residence were derived from the postal code.
The protocol was approved by the Ethics Committee of the University of Antwerp, conditional on the samples being delivered unlinked and anonymous to the investigators.
Serology testing
The samples consisted of residual serum, or heparin or EDTA plasma and were stored frozen (–20°C) until testing. They were analysed with commercially available enzyme-linked immunosorbent assays (ELISAs) in the virology laboratory at the Scientific Institute of Public Health (IPH), Brussels, Belgium, with a semi-automatic pipetting system. The IgG titres against MMR and D were measured for all ages. In the <40 years age group, anti-D was measured in a random subsample of 300 subjects stratified by age as seronegativity was expected to be rare in this age group. Anti-T was measured only in ⩾40-year-olds, as they were more likely to be not yet or less targeted by universal vaccination in childhood. The kits used for detection of MMR antibodies were the anti-measles IgG Enzygnost® kit from Dade Behring (Germany), the anti-mumps IgG ELISA kit manufactured by Hycor Biomedicals (Germany), and the anti-rubella IgG ETI-RUBEK-G PLUS kit from Diasorin (Italy). Sensitivity as reported by the manufacturer was 99·6%, 100% and ⩾99·0% for measles, mumps and rubella, respectively, whereas specificity was reported as 100%, 88% and ⩾96·1%, respectively. For diphtheria and tetanus, the anti-D kit from Hycor and the anti-T kit from Novatec (Germany) were used, and their sensitivity and specificity were both reported to be 94% for D and >95% for T.
Samples were categorized as seropositive, equivocal or seronegative for each antibody according to the cut-off values proposed by the manufacturer. Quantitative titres were obtained from the optical density (OD) values as specified by the manufacturer. Anti-D and anti-T titres <0·01 IU/ml were considered as seronegative whereas titres >0·1 IU/ml were considered as seropositive. Anti-D seropositives were considered seroprotected [14], whereas only those with anti-T >0·15 IU/ml were considered seroprotected against tetanus since titres <0·16 IU/ml with standard ELISA have been reported to be frequently overestimated [15, Reference Gergen16]. For measles IgG, titres <0·15 IU/ml and >0·35 IU/ml, were considered seronegative and seropositive, respectively, for mumps IgG these lower and upper cut-offs were <8 and >12 arbitrary units (AU)/ml, respectively. Correlates of protection have not clearly been defined for these diseases. For rubella IgG, titres ⩽9 IU/ml were considered seronegative and titres ⩾11 IU/ml seropositive according to the test manual, but titres ⩾10 IU/ml were considered seroprotective according to current consensus [Reference Skendzel17].
Statistical analysis
Exact binomial 95% confidence intervals were calculated per age group. For all studied infections except tetanus, the prevalence of seronegativity, seropositivity and equivocal results was standardized for age and gender according to the Belgian population structure aged <66 years in 2006, based on National Registry data [18].
Logistic regression evaluated the effect of age, gender and region and their two-way interactions upon the serostatus (negative, equivocal, positive) for each pathogen independently. Since the effect of the predictors, especially age, upon the serostatus of measles, mumps and rubella was expected to be different in age groups targeted by universal MMR vaccination compared to non-targeted age groups, this analysis was performed per age group (2–22 years vs. older). Within the MMR-targeted subgroup, an extra factor indicated if the target age for the second dose of MMR (MMR2) had been reached (from age 10 years onwards). Multinomial or binary models were used according to the number of outcome categories that were taken into account. Final models were selected using stepwise backward selection, omitting terms with a P value >0·1. Significance was defined as a P value <0·05. SPSS version 15.0 (SPSS Inc., USA) was used for the regression analysis.
RESULTS
Sample collection
A total of 3974 samples were collected. The sampling date ranged from January 2006 to October 2007; 91·4% of the samples were from 2006. Stratification for age and gender was respected, overall 50·2% were males. Based upon the postal codes, 57·3% of samples were from people living in Flanders, 33·0% from Wallonia and 9·7% from Brussels. This is very close to the distribution in the total Belgian population in 2006, which consisted of 58% Flemish, 10% Brussels and 32% Walloon people based on data of the National Registry [18]. Proportionality was also achieved on the provincial level.
Measles, mumps and rubella antibody results
A total of 3323 (85·6%) samples were seropositive (>0·35 IU/ml), 257 (6·6%) were equivocal and 304 (7·8%) were seronegative (<0·15) for measles. When standardized for age and gender, these proportions were 92·2%, 3·9% and 3·9%, respectively.
The World Health Organization (WHO) targets for elimination of measles [19] were reached in children aged <5 years (<10% seronegative) and in adults aged >24 years (<5% seronegative), while >10% aged 5–9 years and >5% aged 10–24 years were seronegative.
For rubella, 3173 (81·0%) samples were seropositive (⩾11 IU/ml), 117 (3·0%) were equivocal and 627 (16·0%) were seronegative (⩽9 IU/ml). After standardization for age and gender, these proportions were 87·4%, 2·2% and 10·4%, respectively.
In women of childbearing age (15–39 years), 13·8% (95% CI 12·1–15·6) were seronegative and 15·0% (95% CI 13·3–16·9) had a non-protective rubella-antibody level (<10 IU/ml).
For mumps, 3156 (81·6%) samples were seropositive (>12 AU/ml), 217 (5·6%) were equivocal and 496 (12·8%) were seronegative (<8 AU/ml). After standardization for age and gender, these proportions were 87·6%, 4·4% and 8·0%, respectively.
Figure 1 depicts the percentage of seronegativity, respectively, for measles, rubella and mumps antibodies according to age, whereas the distribution by gender and region is summarized in Table 1. In children aged between 2 and 10 years the percentage of seronegativity against any of the three diseases gradually increased, whereas it fluctuated around a lower level between the ages of 12 and 21 years. In adults aged >22 years the percentage of seronegativity decreased gradually for measles and mumps, and reached zero in the 40–44 years and 55–59 years age groups, respectively. On the other hand, for rubella an initial decrease was found in age groups from 22 to 29 years, after which the proportion of seronegatives fluctuated around 4·9%.
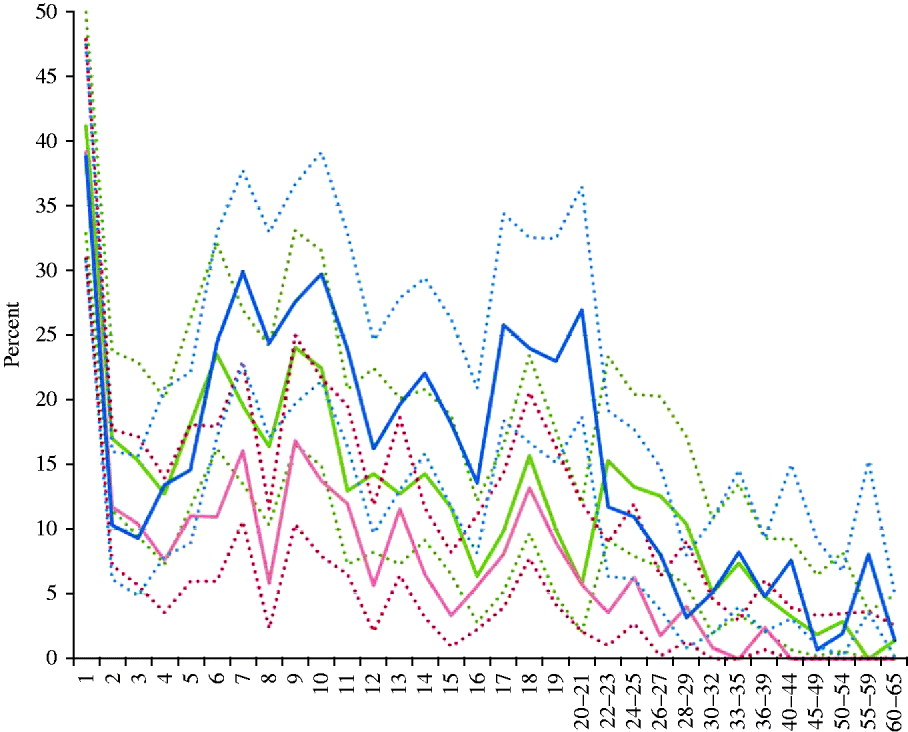
Fig. 1. Prevalence (%) of seronegativity against measles, mumps and rubella per age group (years), Belgium, 2006. Solid lines represent prevalence of seronegativity for measles antibodies (<150 mIU/ml) (red), mumps antibodies (<8 AU/ml) (green) and rubella antibodies (<9 IU/ml) (blue); dashed lines represent upper and lower limits of the exact binomial 95% confidence interval.
Table 1. Prevalence of seronegativity (%) by region, gender and age, for measles, mumps, rubella, and diphtheria, in Belgium, 2006
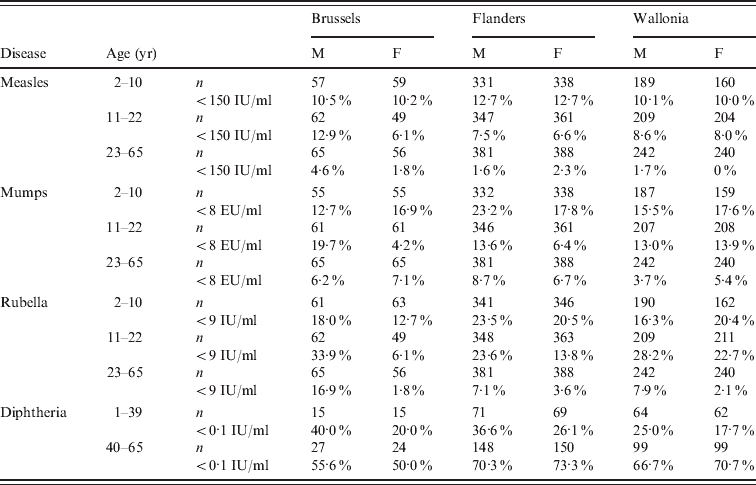
EU, Arbitrary ELISA units; M, male; F, female.
n, Total number of samples with a result for the respective antibody.
Regression analysis was applied as described in the Methods section. For each pathogen independently, multinomial regression was used to calculate odds ratios of seronegatives and equivocals, respectively, to seropositives. In view of the extremely low numbers of equivocal samples for rubella in those aged >22 years (n=15) we had to use binary regression to evaluate rubella serostatus in this age group.
Within the 2–22 years age group, who had been targeted with MMR, significant findings for measles were that the odds of being seronegative decreased with age (OR 0·97, P=0·023), whereas the odds of being equivocal increased with age (OR 1·05, P<0·001) and were higher in Flanders than in Wallonia (OR 1·73, P=0·001). For mumps, girls were less frequently seronegative than boys, and this gender effect was stronger in Flanders than in Wallonia (OR 0·49, P=0·007). Children aged >10 years, who were targeted with MMR2, had lower odds of being seronegative for mumps than younger children who had not yet been offered MMR2, and this was also expressed more in Flanders than in Wallonia (OR 0·45, P=0·002). Furthermore, the odds of being seronegative for mumps increased with age in children aged 2–9 years, but decreased in older children (OR 0·86, P<0·001). With respect to equivocal mumps results, odds were higher in Brussels than in Wallonia (OR 2·54, P=0·036) and increased more with age in girls than in boys (OR 1·06, P=0·033).
For rubella, girls were less frequently seronegative than boys, and this gender effect was larger in Brussels than in Wallonia (OR 0·28, P=0·003). In Flanders compared to Wallonia, odds of being seronegative for rubella were lower in children who were targeted with MMR2 than in those who were not (OR 0·15, P<0·001), and increased more with age in both MMR-targeted groups (OR 1·12, P=0·002). In all regions, children who were targeted with MMR2 were less frequently seronegative for rubella if they were female (OR 0·52, P=0·002) or older (OR 0·82, P<0·001), both when compared to children who were not yet targeted with MMR2. Equivocal rubella results were not significantly associated with any factor.
Within the non-MMR-targeted subgroup (aged >22 years), increasing age was associated with decreasing odds of seronegativity for measles and mumps (OR 0·86 and 0·33, respectively, P<0·001 for each) and also for rubella, but with a gender interaction: females were less frequently seronegative for rubella than males, and this gender effect decreased with age (OR 1·08, P<0·001). This probably reflects the former monovalent rubella vaccination that was offered to women up to age 45 years. Odds of being rubella seronegative were higher in Brussels than in Wallonia (OR 5·98, P=0·009).
Diphtheria and tetanus antibody results
The anti-D titre was >0·1 IU/ml in 386 (45·7%) samples, <0·01 IU/ml in 13 (1·5%) samples and in between in the remaining 445 (52·7%) samples. After standardization for age and gender, these proportions were 55·2%, 1·3% and 43·5%, respectively.
As illustrated in Figure 2, the proportion with anti-D >0·1 IU/ml was >70% in all age groups aged <30 years and reached its highest level at 80% in adolescents (10–19 years). However, the seroprotection rate decreased steeply with age in subjects aged >30 years, to a minimum of 20% in the 55–59 years age group. Susceptible persons (<0·01 IU/ml) were found to be rare at any age, with a maximum of 3·3% in the 30–34 years age group.
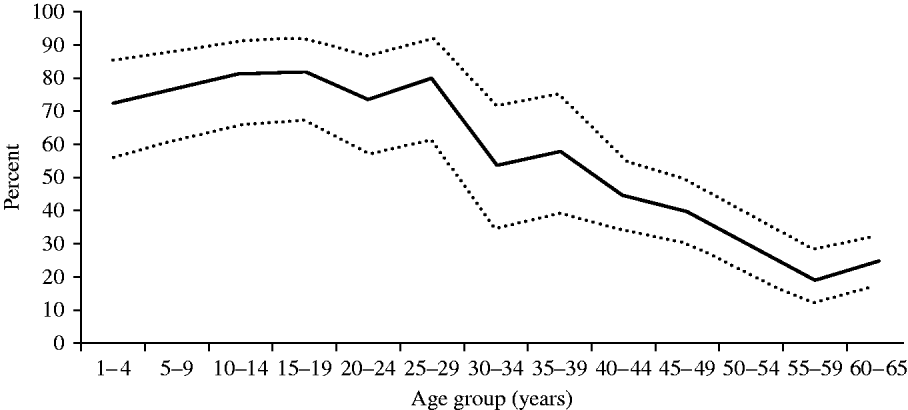
Fig. 2. Proportions seroprotected against diphtheria by age, Belgium 2006. Solid lines represent prevalence of seroprotective anti-D titre (>0·1 IU/ml); dashed lines represent upper and lower limits of the exact binomial 95% confidence interval for the prevalence in the total population.
Binary logistic regression (using 0·1 IU/ml as cut-off) found significantly less seroprotection against D (>0·1 IU/ml) in people living in Flanders compared to Brussels (OR 0·54, P=0·02), as well as a gender-dependent effect of age with more loss of seroprotection with age in females than in males (OR 0·98, P=0·025).
Anti-T was evaluated in subjects aged ⩾40 years, and was found to be >0·1 IU/ml in 495 (90·7%) of them, and within the 0·01–0·1 IU/ml range for the remaining 51 (9·3%). The level of 0·16 IU/ml, which was used as a limit for seroprotection in this study, was reached in 476 (87·2%) of the evaluated subjects. As shown in Table 2, the proportion of adult blood donors with anti-T >0·15 IU/ml decreased slightly with age to a minimum of 79·6% in the 60–65 years age group.
Table 2. Seroprotection against tetanus in ⩾40-year-olds in Belgium, by region, gender and age
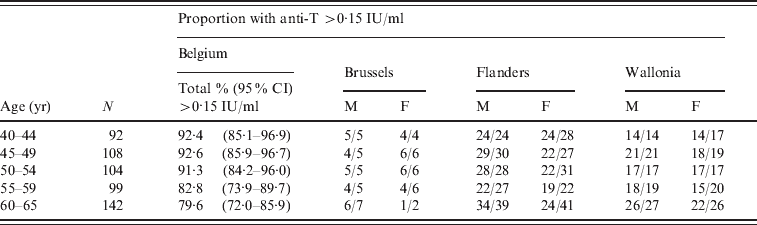
N, Total number of samples; 95% CI, exact binomial 95% confidence interval; M, male; F, female.
Binary logistic regression (using 0·15 IU/ml as cut-off) found significantly less seroprotection against T (>0·15 IU/ml) in females (OR 0·23, P<0·001), and a decrease of seroprotection with age that was higher in Flanders than in Wallonia (OR 0·97, P=0·002).
DISCUSSION
The aim of this serological survey was to evaluate the current age-specific seroprevalence within the Belgian population for infectious diseases against which vaccination had been widely implemented for decades in infancy as well as at older ages. The prevalence of seropositivity in early childhood adds useful information to vaccination coverage data, as in Belgium coverage surveys have only been performed at a regional level and not simultaneously in the respective regions. Furthermore, age-specific seronegativity provides an indication for remaining or accumulating susceptibility at the population level. Many European countries have a similar vaccination schedule and disease epidemiology for these diseases as Belgium (see euvac.net). The relevance of the results therefore may exceed the local level.
Measles, mumps and rubella
The prevalence of seronegativity for measles and mumps within the general population was low, at 3·9% and 8·0%, respectively. However, the WHO's targets for elimination of measles were not reached in the population aged between 5 and 24 years [19]. This confirms the findings of a previous serosurvey performed in 2002 as part of the European Seroepidemiology Network 2 (ESEN2) EU project [Reference Andrews11].
These findings are not unexpected, as infant MMR coverage has been suboptimal until recently. The first time this coverage rate surpassed 95% was in Flanders in 2008 [Reference Boonen20]. In Brussels and the Walloon region coverage was still <95% during the most recent coverage surveys in 2006 and 2009, respectively [Reference Swennen and Robert8]. Coverage data for the second MMR dose were even <90%, which was exceeded only in Flanders in 2008 [Reference Boonen20]. The estimated incidences of measles and mumps declined rapidly during the first 10 years of universal vaccination, and were between 1/103 and 1/104 in 1996–1999 based on a sentinel general practioners' network [Reference Vander Veken and Van Casteren21, Reference Beutels22]. Since 2002 a new surveillance system (Pedisurv), mainly based on a sentinel paediatricians' network, has registered measles and mumps cases. This surveillance reveals that local outbreaks of measles and mumps still occur, and measles incidence is still >1/105. However, age-related seroprevalence (Fig. 1) suggests that chances for natural boosting have markedly decreased, as seronegativity accumulates in the 6–10 years age group who have been targeted by the first vaccine dose but not yet by the second. This phenomenon was also noticed in other European countries and has been related to waning antibody titres [Reference Amela, Pachon and de Ory23–Reference Tischer and Gerike25]. In the 2–4 and 14–16 years age groups, seronegativity was found to be lowest (Fig. 1). These age groups had most recently received the first or second MMR dose, recommended at 1 and 10–13 years, respectively. However, it should be noted that a recent local outbreak of measles in a Jewish community with at least 137 cases did not give rise to an epidemic in the general population. This would have been due to sufficiently high numbers of immune persons in the contacts of cases, such that their susceptible contacts in turn were protected by herd immunity [Reference Lernout26].
In the case of measles and mumps, EIA seronegativity is no synonym for susceptibility, because no protective titre has been established. Plaque reduction neutralization (PRN) values >120 were found to confer clinical protection against measles in an outbreak study [Reference Chen27], but EIA values do not perfectly correlate with PRN [Reference Ratnam28], and seronegative children have been found protected when exposed to measles [Reference Samb29].
Elimination of measles from Belgium should be achievable with the current two-dose approach, but more efforts are needed such as increasing the coverage of the second MMR dose in adolescents and young adults. A higher coverage with the second MMR dose would also enhance mumps control. Waning immunity and breakthrough mumps infections after a single MMR dose have been demonstrated [Reference Vandermeulen30, Reference Briss31]. In Belgium, the majority of mumps cases are reported in children that have not yet been targeted with the second dose, between ages 1 and 9 years, although 1/3 cases are aged ⩾10 years [Reference Sabbe and Hue32].
The prevalence of rubella seronegativity in children varied from 10% to 30% in our study, which implies a risk for outbreaks in children who could infect unprotected women during pregnancy. Moreover, the proportion of women at childbearing age with an unprotective antibody level for rubella (<10 IU/ml) was high at 15·0%. Similar findings were reported from the 2002 serosurvey, and contrasted with the majority of other European countries [Reference Nardone12]. Only one case of congenital rubella syndrome (CRS) was reported in Belgium during the past decade [Reference Vander Veken and Van Casteren21, Reference Sabbe and Hue32] and reported rubella incidence is low, but underreporting is possible as registration is not mandatory and active surveillance has only existed for CRS since 2007. On the other hand, an overestimation of seronegativity cannot be excluded taking into account that the commercial ELISA used for detection of rubella in both the current and the 2002 study was shown to be less sensitive than other ELISAs used within ESEN2. Furthermore, in studies on the response to MMR vaccine, the rubella response was generally found to be the strongest whereas it seems to be the weakest in this study [Reference Plotkin, Reef, Plotkin, Orenstein and Offit33, Reference Vyse34]. In Belgium the RA 27/3 vaccine strain has always been used, which is known to confer excellent protection [Reference Plotkin, Reef, Plotkin, Orenstein and Offit33]. A small-scale study on rubella antibody titres in 310 Flemish participants aged 6–17 years in a vaccine trial performed in 2000 found only 7·8% seronegatives using a Microparticle Rubella IgG EIA (AxSYM, Abbott, USA) [Reference Vandermeulen35]. When we applied the same standardization as used within ESEN2 [Reference Kafatos, Andrews and Nardone36, Reference Tischer37] on the current 2006 data for rubella, the unprotected women of child bearing age decreased to 11·5% (95% CI 9·8–13·0), which is similar to the 13·4% (95% CI 10·9–15·9) reported from the 2002 serosurvey [Reference Nardone12]. The standardized seronegativity in children aged <15 years was 11·5%, which is very close to the 12·8% reported in 2002. However, these adjusted proportions are still higher than in the majority of the European countries that participated in ESEN2, and far above the WHO target of 5% susceptibility in women of childbearing age [19].
The regional and gender-specific differences that were found for mumps and rubella probably reflect the historical regional differences in MMR coverage as well as delay of replacement of monovalent rubella vaccine by MMR2 in pre-adolescent girls. The lower prevalence of seronegativity for mumps and rubella in children targeted with MMR2 in Flanders corresponds with the higher MMR coverage for both doses in this region, and vaccine-induced antibody responses against MMR have been reported to be higher in females [Reference Thomas and Moridani38]. The contrasting finding that region and gender were not associated with measles seronegativity in both targeted and non-targeted age groups, could reflect that the level of remaining natural measles circulation, altthough low, is still higher than for mumps and rubella. Indeed, the level of protection required to fully prevent circulation is supposed to be the highest for measles [Reference Anderson and May39].
The prevalence of seronegativity for measles, mumps and rubella was generally found higher in the MMR-targeted age groups than in the non-targeted groups. Hence, a future increase of seronegativity within young adults can be expected.
Diphtheria and tetanus
The diphtheria seroprotection rate (>0·1 IU/ml) in the general population was suboptimal at 55·2% but was >70% in children, which should confer herd immunity thereby preventing outbreaks in adults [14]. In fact only one case of diphtheria has been reported in Belgium since 1980. In the 40–65 years age group, the seroprotection rate for tetanus was higher than for diphtheria but still suboptimal at 87·2%. This age group was suspected to be the least protected against tetanus since none of them had been targeted by the adolescent dT booster and those aged >49 years had not even been targeted in infancy; however, men were vaccinated during military service (in early adulthood up to 1995) and men are known to be better reached by adult dT boosters due to gender-specific differences in professional and leisure activities, which is reflected in their higher immunity for tetanus. A recent study of tetanus seroprotection in an urban emergency department in Belgium showed a similar age and gender preference, and found that being followed by occupational medicine had a clear positive effect [Reference Stubbe40].
Compared to the diphtheria seroprevalence study performed in Flanders in 1993–1994, before the recommendation to boost adolescents and adults with d-containing vaccines, diphtheria seroprotection has markedly increased and the susceptibility rate (1·3% <0·01 IU/ml) has decreased accordingly. These rates were previously estimated at 43% and 32%, respectively, based on 1679 samples collected in hospitals in 1993–1994 and analysed using an in vitro neutralization test on Vero cells [Reference Mathei10]. When comparing age-specific rates, susceptibility was much lower in all age groups in the current study, and some age shifts could clearly be identified. In 1993–1994, seroprotection decreased from the age of 15 years onwards down to 21·4% in the 35–44 years age group, and rose only slightly to 38·4% in the 55–64 years group and 32·7% in the oldest age group. In the current study, the decrease started only at age 25 years and reached similar low levels only in the 55–65 years group. This age group has not been targeted by the universal vaccination that started in 1959, and immunity from natural infection was probably also limited within this group as the incidence of diphtheria was already declining 10 years before the vaccination started [Reference Mathei10]. Natural immunity could still exist in people aged >65 years, but those were not evaluated in this study. Obviously, within the evaluated age groups the booster dT offered free of charge by the school medicine system since 1995, as well as the simultaneously launched recommendation to use combined dT vaccines instead of monovalent T vaccines in adults whenever a tetanus vaccination was indicated, have ameliorated the seroprotection rates of adults against diphtheria. Monovalent tetanus vaccines have not been available in Belgium since 2003, supporting the above-mentioned recommendation. Further improvement of seroprotection against D and T should be feasible in Belgium, as in Finland >70% seroprotection was achieved against both diseases in the population aged ⩽50 years [Reference Olander41].
General considerations and concluding remarks
This serosurvey was based on residual samples which could have caused bias, especially in the adult population. The infant samples were retrieved from hospitals using specific criteria to minimize selection of immunosuppressed patients, a method which has been shown to provide estimates of immunity against vaccine-preventable diseases that are comparable to those from a random cluster survey and that has been successfully applied for serosurveillance in other countries [Reference Osborne1–Reference Gabutti3, Reference Andrews11, Reference Nardone12, Reference Kelly42]. The adult population exclusively consists of blood donors, a specific group which could be more inclined to receive vaccinations than the general population.
On the other hand, commercial ELISAs meant for diagnostic use are known to optimize specificity at the expense of sensitivity, and therefore tend to underestimate seroprevalence in an epidemiological setting. An approach that has been proposed to overcome this cut-off problem is to apply mixture modelling on the antibody titres to distinguish and quantify subpopulations with different states of immunity [Reference Vyse34, Reference Rota43].
Despite these limitations, the results of this serosurvey in Belgium indicate suboptimal protection against measles, mumps and rubella in children and young adults, and against diphtheria and tetanus in older adults, in the face of universal free-of-charge vaccination for 20 years for MMR and 10 years for dT. A risk of outbreaks and CRS remains, which underlines the need for active surveillance and improvement of immunization coverage. Efforts should focus on enhancing the coverage of the second dose of MMR, also beyond the pre-adolescent age. Implementation of regular dT boosters has reached young adults, but should further be endorsed in those aged >40 years. Currently, pertussis-containing adult dTpa boosters are implemented in Belgium which creates new opportunities to reach this age group, e.g. when they become parents or grandparents.
ACKNOWLEDGEMENTS
This project was funded by the IPH and the University of Antwerp. P.B. and N.H. acknowledge ‘SIMID’, a strategic basic research project funded by the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT), project no. 060081. For sample collection we thank doctors D. Baetens, M. Martin, J. Billiet, S. Vanderschueren, H. De Puydt, W. d'Hoore, A. Mewis, P. Couck, C. Neve, C. Fillee, T. Ledant, A. Dediste, C. Potvlieghe, P. Goffinet and C. Pacco from the collaborating diagnostic laboratories and A. De Smet and M. C. Frère from the regional laboratories of the Red Cross, as well as Mr S. Broodhaers (CEV).
DECLARATION OF INTEREST
None.






