BACKGROUND
Cryptosporidium spp. (Cryptosporidium), a chlorine-resistant protozoan parasite, was first identified as a cause of illness in humans in 1976 [Reference Nime, Burek, Page, Holscher and Yardley1]. Since that time Cryptosporidium has become increasingly recognized as a pathogen responsible for outbreaks of diarrhoeal illness in both immunocompetent and immunocompromised persons. Transmission can be person-to-person, animal-to-animal, animal-to-person, foodborne, and waterborne, and occurs via the faecal–oral route with the ingestion of viable oocysts [Reference Fayer, Morgan and Upton2]. Reported outbreaks have been associated with day-care centres [3–Reference Cordell and Addiss7], contaminated drinking water [Reference MacKenzie, Hoxie and Proctor8, Reference Hayes, Matte and O'Brien9], food [Reference Millard, Gensheimer and Addiss10–12], and recreational water facilities (both chlorinated and non-chlorinated facilities) [Reference Baker, Russell, Roseveare, O'Hallahan, Palmer and Bichan13–Reference Sundkvist, Dryden, Gabb, Soltanpoor, Casemore and Stuart19].
In the past several years there has been an increasing trend in the number of recreational water-associated outbreaks of cryptosporidiosis suggesting a need for public health efforts to be focused on improving the understanding and prevention of this illness in aquatic facilities. Swimming is enjoyed by adults and children alike, and in the United States swimming is the second most popular exercise activity with >360 million annual visits to recreational water venues [20]. The chlorine resistance of Cryptosporidium, the frequent exposures to swimming, high bather densities, and heavy use by diaper-aged children who have a high prevalence of Cryptosporidium [Reference Meinhardt, Casemore and Miller21] have all contributed to this increasing trend in recreation water facility-associated outbreaks. In addition, improved recognition and increased reporting of such outbreaks may play a role in contributing to this increasing trend.
In this paper we report an outbreak of cryptoporidiosis associated with a recreational waterpark where transmission appears to have occurred despite a well-maintained and managed swimming environment.
METHODS
On 13 August 2001, the Illinois Department of Public Health (IDPH) was notified of a cluster of three cases of diarrhoeal illness associated with exposure to a recreational waterpark in central Illinois. Cryptosporidium was identified in stool samples of ill patients. Following the collection of pool water samples, the waterpark was closed and all pools within the waterpark were hyperchlorinated (to maintain free chlorine levels of 20 ppm for 8 h) to inactivate Cryptosporidium. An investigation of the outbreak was conducted by Tazewell County Health Department (TCHD), IDPH and CDC to confirm the risk exposure and identify any additional risk factors associated with illness. This research was conducted in compliance with all applicable federal regulations governing the protection of human subjects' research.
Case definition
A laboratory-confirmed case was defined as a person living in or visiting central Illinois between 1 July and 31 August 2001 with a positive Cryptosporidium stool test and at least one symptom of either diarrhoea, vomiting, or abdominal cramps. A clinical case was defined as a person living in or visiting central Illinois between 1 July and 31 August 2001, with ⩾1 day with ⩾3 loose (or watery) stools in 24 h. A control was defined as a person living in or visiting central Illinois between 1 July and 31 August 2001, without any symptoms of illness during the same time period. Controls were matched by age and residential neighbourhood.
Epidemiological investigation
A descriptive study and two case-control studies (community-based and waterpark-based) were conducted. Through the local media and community networks, ill persons were encouraged to contact TCHD. In order to identify other potentially exposed persons, case-patients were asked if there were any other ill persons in their household or if anyone attending the waterpark with them was ill. Matched controls were identified by the following hierarchy of methods: first, through case-patients (who were asked to identify another healthy person within 5 years of their age), secondly through the local reverse-telephone directory based on residential address of case-patients, and thirdly through canvassing local schools and community groups. The majority of controls were identified by the first and second methods. A subset of the case-patients and matched controls identified for the community-based case-control study were included in the waterpark-based study.
A standardized telephone-administered questionnaire was used to collect information from potential case-patients and controls regarding demographics, symptoms of recent illness (within the previous month), type of medical attention sought, and diagnostic tests performed. Case-patients ⩾12 years of age were interviewed directly while parents or guardians of case-patients <12 years of age were interviewed as a proxy. In addition, case-patients were asked if they had recent exposure to persons with similar illness (or for controls, persons with diarrhoea). Based on the case definition and the incubation period for cryptosporidiosis, details of known risk exposures during the 2 weeks prior to the onset of symptoms for case-patients (and during a 2-week period between 1 July and 31 August 2001 for controls) were recorded. These included consumption of food and drinking water, contact with young children, recent travel, and any recreational water activities. When a recreational water exposure was reported, specific waterpark-related behaviours were explored. These included time spent in the water, swimming in certain pools within the park, getting water in the mouth, swallowing water, diving into water, having the head under water, using the waterslide, having water splashed into the face, and consumption of food and beverages at the waterpark.
The community-based, case-control study enrolled all laboratory-confirmed case-patients and matched controls. The waterpark-based, case-control study limited enrolment to laboratory-confirmed case-patients and controls with exposure to the waterpark on 3 and 4 August, the days with the highest reported case-patient attendance.
Data were entered using Epi-Info, version 6.04 (CDC, Atlanta, GA, USA). Data analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA). Odds ratios were calculated using conditional logistic regression for matched pairs and χ 2 tests were used to test for statistical significance. Two-sided P values <0·05 were deemed statistically significant.
Laboratory investigation
Ill persons were encouraged to submit formalin-preserved stool specimens for Cryptosporidium testing. The local hospital laboratory in Tazewell County, IDPH laboratory, and CDC, tested stool for Cryptosporidium using the ProSpecT Cryptosporidium Microplate Assay (Alexon-Trend Inc., Sunnyvale, CA, USA), acid-fast staining, and microscopy. Positive specimens were concentrated using the formalin-ethyl acetate technique and then confirmed by the Merifluor Cryptosporidium/Giardia direct fluorescent assay (DFA) (Meridian Bioscience Inc., Cincinnati, OH, USA). Potassium-dichromate-preserved stool specimens were tested by CDC using DFA or polymerase chain reaction (PCR) analysis. A small subunit rRNA-based polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) tool was used for genotyping [Reference Xiao, Bern and Limor22] and a PCR-sequencing tool based on the 60 kDa glycoprotein (GP60) was used for subtyping [Reference Alves, Xiao, Sulaiman, Lal, Matos and Antunes23].
Environmental investigation
The initial environmental assessment attempted to determine likely routes for parasite contamination of the pool and subsequent transmission, and to identify any immediate remediable problems. The environmental investigation included site visits to the waterpark, assessment of the procedures and practices of the facility, evaluation of water sources, and the physical environment surrounding the waterpark. Information on the occurrences of faecal accidents and the responses of the pool management were reviewed together with water chlorination, pH levels and filter backwash records and procedures. Information was collected concerning waterpark group rentals, foods served at the concessions stand, dates and hours of operation, bather load, water clarity, pool operation and maintenance records, and pool design (including filters, turnover, and chlorination equipment). Water-quality data and treatment processes of the local water-treatment facility, which distributes water to the community in which the waterpark is located, were also reviewed.
Prior to hyperchlorination of the pools at the waterpark on 13 August 2001, pool water (10 l from the toddler/wading pool, 10 l from the lap pool, and 10 l from the Lazy River) and backwash material (10 l of a mix of water and diatomaceous earth filter media from each filter) were collected for testing. These samples were tested at CDC for Cryptosporidium using USEPA Method 1622, which detects oocysts by immunofluorescence after concentration of the water sample using immunomagnetic beads to capture oocysts [24]. Samples also were tested by PCR [Reference Xiao, Alderisio, Limor, Royer and Lal25, Reference Xiao, Singh, Limor, Graczyk, Gradus and Lal26] and with an electrochemiluminescence (ECL) antigen test [Reference Lee, Johnson and Call27].
RESULTS
Descriptive study
From August 13 to 30 September 2001, a total of 358 case-patients (281 clinical cases and 77 laboratory-confirmed) were identified by the investigation team. Case-patients were predominantly children (77·9% <18 years old; median 9 years; range 1–72 years). Sixty-nine per cent of children were <12 years of age and were interviewed by a proxy (parent or guardian). Among the laboratory-confirmed case-patients, the most commonly reported symptoms were diarrhoea (94·8%), abdominal cramps (88·0%), and nausea (76·4%). Symptoms among clinical case-patients were similar (Table 1). More than half (63·2%) of the laboratory-confirmed case-patients sought medical care, and seven case-patients (9·0%) were hospitalized as a result of their illness. Hospitalized case-patients were 6–71 years of age. The epidemic curve depicting clinical and laboratory-confirmed cases by date of symptom onset shows that transmission was occurring from mid July to the end of August (Fig. 1).
Table 1. Clinical symptoms and hospitalization from cases identified in an outbreak of cryptosporidiosis, Tazewell County, Illinois, 2001
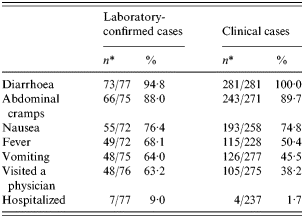
* Denominator varies due to missing responses in questionnaire.

Fig. 1. Epidemic curve showing laboratory-confirmed case-patients (▪, n=77) and clinical case-patients (□, n=281) by date of symptom onset.
Community-based case-control study
There were 77 case-patients and 77 matched controls enrolled into the community-based case-control study. Exposures examined for association with cryptosporidiosis are presented in Table 2. Swimming at the waterpark was strongly associated with cryptosporidiosis (OR 16·0, 95% CI 3·8–66·8). Having any contact with a day-care centre (visiting or working at a day-care centre or a child being in day care) was also associated with illness (OR 6·0, 95% CI 1·3–26·8). There appeared to be no statistically significant association between consumption of drinking water from any source (bottled, municipal or a private well) and illness.
Table 2. Percent exposed, odds ratio (OR), and 95% confidence intervals (CI) for cryptosporidiosis risk factors evaluated in the community case-control study, Tazewell County, Illinois, 2001

* All exposures occurred in the 2 weeks prior to symptom onset (case-patients) or in a 2-week period between 1 July and 31 August 2001 (controls).
† Denominator varies due to missing responses in questionnaire.
‡ Visited a day-care centre, or worked at a day-care centre, or child was in day care.
Waterpark-based case-control study
Fifty case-patients and 50 matched controls were enrolled in the waterpark-based case-control study. Behaviours significantly associated with cryptosporidiosis were getting pool water in the mouth (OR 6·0, 95% CI 1·3–26·7) and swallowing pool water (OR 4·5, 95% CI 1·5–13·3) (Table 3). There was no significant association with swimming in a particular pool, or spending more than one cumulative hour in the pool water.
Table 3. Percent exposed, odds ratio (OR), and 95% confidence intervals (CI) for risk factors for cryptosporidiosis evaluated in the waterpark-based case-control study, Tazewell County, Illinois, 2001

* Denominator varies due to missing responses in questionnaire.
Laboratory investigation
Testing of formalin-preserved stool specimens submitted by symptomatic patients identified 77 positive specimens (i.e. laboratory-confirmed case-patients). In addition, 22 potassium-dichromate-preserved stool specimens from symptomatic patients were examined. Ten (45·5%) of these were positive by DFA and PCR for Cryptosporidium. Genotyping revealed Cryptosporidium hominis (this species only infects humans) in all PCR-positive individuals. Subtyping analysis indicated that all of the 10 C. hominis-positive samples had a parasite belonging to the subtype family Ia [Reference Strong, Gut and Nelson28].
Results of the pool-water grab samples and backwash samples collected from the waterpark are presented in Table 4. All samples were PCR negative. USEPA Method 1622 revealed Cryptosporidium oocysts in the grab samples from the toddler/wading pool and lap pool but not the Lazy River, and backwash from the toddler/lap/slide (not the river/tree house backwash) filter system. ECL was positive for both samples of filter backwash.
Table 4. Water and diatomaceous earth (DE) backwash samples, results from USEPA method 1622, ECL and PCR testing

n.t., Not tested.
Environmental investigation
Figure 2 shows the layout of the main water features of the waterpark. The waterpark consisted of a toddler/wading pool (i.e. zero entry-level pool transitioning to a depth of 18 in.), a lap pool (depth of 3 ft 6 in. to 5 ft 0 in.), a ‘Lazy River’ (constant depth along length of 2 ft 6 in.), one major slide/plunge pool (depth of 2 ft 0 in. to 3 ft 6 in.) and three smaller slide/plunge pools (depth 18 in.). There was no designated diving pool. Child-oriented water features included a tree house climbing structure and bucket drop and several fountains and water sprays.

Fig. 2. Layout of waterpark associated with Cryptosporidium outbreak, Illinois, 2001.
Male and female restrooms, both with showers and diaper changing stations, were available within the complex. A concessions outlet serving drinks and snacks was also located within the pool area. The pool complex was surrounded by a 6 ft high fence on three sides and a building/entrance to the complex on the fourth side. The waterpark was open every day of the week, with restricted access for groups (including swimming clubs, lessons, and private functions) in the early mornings and late evenings.
The waterpark employed two diatomaceous earth (DE) filtration systems; each with automated chlorination pumps using liquid sodium hypochlorite, 12·5%. One DE system filtered water from the toddler pool, lap pool and slide/plunge pools (estimated water volume 200 000 gallons; turnover 3·4 h), while the other system filtered water from the Lazy River, tree house and three small plunge pools (estimated water volume 255 000 gallons; turnover 2·8 h). All the pools at the waterpark are heated to a set point of 28°C. During peak times the waterpark generally has a daily attendance of ∼1500 bathers. On 3 August there were 1023 and on 4 August, a Saturday, there were 1836 bathers.
The pool log records indicated that pH levels of the chlorinated pool water were monitored 2–3 times per day and recorded daily. No cyanurates were used. Waterpark records indicated that one faecal or vomit accident occurred approximately every 2 days. For 3 August there were two accidents recorded (one faecal and one vomit both in the lap pool) and for 4 August there were no events recorded, although further questioning of staff revealed there had been three faecal accidents on that day. No distinction in the records was made between formed stool and diarrhoeal episodes.
Routine management of faecal accidents at the waterpark appears to have followed CDC recommendations for formed stool [29]: removal of organic material, check of free chlorine levels and if free chlorine <2 ppm, then addition of chlorine to bring level the chlorine level >2 ppm, and closure of the pool to patrons for 20–30 min. No diarrhoeal accidents were reported. Chlorine levels on 3 and 4 August were recorded as being >2 ppm in the lap pool, toddler pool and the Lazy River. For all the slide/plunge pools, chlorine levels were <2 ppm (within acceptable levels of chlorine residual, according to the Illinois Swimming Pool and Bathing Beach code [30]). The pool records also mention the use of a vacuum for cleaning the pool floor in some instances after a faecal accident, a technique not recommended by CDC as no uniform recommendations for disinfection of vacuum systems are available [29]. Staff reported no maintenance problems during the immediate period prior to the outbreak. However, no official maintenance logs were kept.
Water-quality data maintained at the local water treatment facility that supplied the waterpark and the surrounding community indicated the system was operating within state regulations. The information collected on food served at the pool and the source of that food, did not reveal any additional information considered pertinent to the investigation.
DISCUSSION
The outbreak described is the largest reported outbreak of cryptosporidiosis in Illinois. In recent years, Illinois has reported between 90 and 126 cases of cryptosporidiosis annually [31]. The case-patients associated with this outbreak resulted in the state reporting a total of 483 cryptosporidiosis cases in 2001, the highest since reporting began in 1995. The results of the epidemiological studies combined with the results from the laboratory analyses of human specimens and environmental samples suggest that the source of the outbreak was human, and was likely to have been the result of a diarrhoeal accident from an infected individual swimming at the waterpark.
The epidemic curves illustrate that 9 August 2001 was the most frequent day for symptom onset and that 3 August 2001 was the most frequent exposure day. This is in keeping with the approximate 1-week incubation period of cryptosporidiosis [Reference Navin and Juranek32, Reference Jokipii and Jokipii33] and is further evidence that the outbreak was associated with a visit to the waterpark. The associations between illness and swimming behaviours such as getting water in the mouth and swallowing pool water confirm previously reported risk behaviours [16].
There were a number of limitations and potential biases resulting from the study design. Questionnaires were administered in some cases more than 1–2 weeks after symptoms had resolved, and in the case of children (<12 years of age), questionnaires were administered to parents/caregivers as a proxy for the child. These techniques may have resulted in information bias, however, as case-patients and controls were asked to recall similar time periods in the past, it is unlikely that recall bias would have been differential between the two groups. Similarly, the reporting of behaviours by proxy is unlikely to have influenced the associations found as controls were matched for age. As a result of a variety of publications in the local media related to the outbreak and the implicated waterpark, case-patients and controls may have been aware of the investigation hypothesis, introducing selection bias and possibly contributing to the strength of the association found in the community-based study. Selection bias may also have been introduced as a result of the methodology employed to recruit controls for this study. These selection biases are less likely to have influenced the behavioural associations found in the waterpark-based study.
The presence of Cryptosporidium oocysts in the pool water and backwash samples are further evidence that the waterpark pools contained the parasite and were associated with this outbreak. However, none of the tests performed on these pool samples indicates whether the oocysts detected were infectious. ECL relies on the detection of soluble antigen on the surface of the oocyst and provides a numeric approximation of oocysts present, while USEPA Method 1622 testing involves recognition of primarily intact oocysts. Discordant results between ECL and USEPA Method 1622 on the backwash samples may be explained by the higher sensitivity of ECL than DFA. ECL is an environmental research tool that was originally developed for testing of source or drinking water, and later was applied for oocyst detection in environmental and stool samples. As this method is still being refined and has not yet been standardized, results should be interpreted with caution. In addition, although these water assays are specific they are insensitive and large-volume water testing poses a substantial challenge during the investigation of such outbreaks.
The interconnected design of the pools with shared filtration systems may have contributed to the contamination of multiple pools within the waterpark. Pool water disinfection and filtration systems are widely used to prevent such contamination, however, Cryptosporidium is a chlorine-resistant parasite [Reference Korich, Mead, Madore, Sinclair and Sterling34] and standard swimming-pool chlorine concentrations (1–5 mg/l) are not effective in immediately inactivating Cryptosporidium oocysts. Thus, the organism may survive for days in the pool water [Reference Korich, Mead, Madore, Sinclair and Sterling34]. In addition, due to the small size of the oocysts (4–6 μm) [Reference Fayer, Morgan and Upton2], Cryptosporidium is not effectively removed by filtration systems usually employed in recreational water facilities. Even DE filters that are designed for swimming pool use (because pore size decreases dramatically as the filter removes debris), are not able to fully remove Cryptosporidium oocysts from swimming pool water in a short period of time [Reference Schuler and Ghosh35].
Although this outbreak was not necessarily due to poor pool maintenance, deficient maintenance is reported to be widespread in pools nationwide [36]. The findings of this investigation suggest that even a waterpark complying with existing standards and guidelines may become contaminated with Cryptosporidium oocysts and be involved in an outbreak. The characteristics of the parasite, the limited effectiveness of pool hardware to remove Cryptosporidium oocysts, and the relatively small infective dose of Cryptosporidium [Reference Dupont, Chappell, Sterling, Okhuysen, Rose and Jakubowski37], makes it important to focus on other preventive strategies to further reduce the risk of transmission of cryptosporidiosis. Strategies need to focus on educating and modifying behaviours of pool managers, staff, and patrons to reduce the risk of both contamination of pool water and transmission of infection.
For pool management, the importance of a rapid and adequate response to known and suspected faecal contamination events should be emphasized. Management of faecal and diarrhoeal episodes should adhere to CDC recommended guidelines and should include hyperchlorination for a time period that equates to a contact time (CT) inactivation value of 9600 (e.g. free chlorine levels of 20 ppm for 8 h) [29]. Pool management and staff should ensure the facility complies with current codes and standards of operation and insist on patrons' adherence with regulations and practice of good hygiene when using the pool facilities.
Modification of waterpark design may also help prevent cross-contamination of pools leading to expanded transmission of Cryptosporidium. Such design modifications include separation of the wading/toddler pool filtration system from other facility swimming pools to avoid the potential for cross-contamination and increasing the turnover rate of the toddler pool to ∼30 min. Modification of filtration systems may also be appropriate. As a result of this outbreak and investigation, the waterpark installed an ultraviolet system to complement the existing chlorine pool water disinfection and DE filtration process. Ultraviolet treatment of water effectively inactivates Cryptosporidium oocysts [Reference Korich, Mead, Madore, Sinclair and Sterling34, Reference Corona-Vasquez, Samuelson, Rennecker and Marinas38, Reference Li, Finch, Smith and Belosevic39].
Public education and patron behaviour modification are also critical to reduce the risk of illness. Simple messages to encourage good hygiene are key. Encouraging parents and guardians to insist on the avoidance of swimming while symptomatic with diarrhoea and during outbreaks for 2 weeks following resolution of symptoms, to avoid risk behaviours such as getting pool water in the mouth and swallowing pool water, to ensure frequent bathroom breaks for children and toddlers, and to conduct appropriate diaper changing and hand washing are behaviours that are likely to prevent pool water contamination and transmission of cryptosporidiosis, as well as other illness such as giardiasis and shigellosis.
Early recognition of outbreaks and prompt action by local health departments may help to limit the extent of the outbreak. Early notification by staff at the local hospital, the rapid response by TCHD in recognizing the outbreak, and the subsequent implementation of prevention messages, and hyperchlorination of the pool water may have limited the extent of this outbreak. No secondary laboratory-confirmed cases were identified, nor was there substantial evidence of such cases among the clinical cases, suggesting that efforts to educate the public and disseminate prevention messages such as hand washing and avoidance of swimming while ill and for 2 weeks following illness may have been effective. TCHD also made substantial efforts during the 2001 swimming season to educate the community and to highlight the public health importance of increasing efforts to keep ill persons and persons recovering from enteric illnesses out of recreational waters. Other pools in the community were notified of the outbreak early on as were day-care centres. Without early notification about outbreaks and implementation of prevention measures, satellite pool locations have been known to account for amplification and perpetuation of such outbreaks [16].
Although outbreaks are more likely to be reported in the literature, it is important to consider these in relation to the background of sporadic cryptosporidiosis occurring in the United States, particularly as sporadic cases of cryptosporidiosis are thought to account for the majority of reported cases [Reference Dietz, Vugia and Nelson40]. From a public health perspective, the risk factors associated with sporadic cases are similar to those previously seen among immunocompromised and outbreak-related cases-patients suggesting that health providers and the public need to be aware of the multiple modes of transmission in order to have a substantial impact on the disease [Reference Roy, Delong and Stenzel41].
Protecting patrons and bathers from transmissible illnesses such as cryptosporidiosis is in the interest of both pool management and public health. Public health agencies may want to consider what change in number of cases reported through their local surveillance system requires the implementation of measures to halt further transmission. Achieving a safe swimming environment requires a combination of efforts by individuals and the community. For further CDC recommendations for swimmers, pool operators, and public health officials see ref. [42].
ACKNOWLEDGEMENTS
We thank Judy Conway, Infection Control Practitioner, and staff in the Pekin Hospital Laboratory for their astute recognition and rapid reporting of the first cases to the Tazewell County Health Department (TCHD) and assistance with follow-up activities. We gratefully acknowledge all the staff at TCHD who helped with the administration of our questionnaires, data management, and patient follow-up. We also thank the Illinois Department of Public Health, Division of Laboratories for their assistance with processing of specimens. All funding for this evaluation was provided by Centers for Disease Control and Prevention, Tazewell County Health Department, and the Illinois Department of Public Health.








