Introduction
Plague is a virulent, deadly disease for both wildlife and human populations caused by the bacterium Yersinia pestis. More than 200 species of mammals are known to become infected by Y. pestis, primarily rodents, but also carnivores (Biggins & Kosoy Reference Biggins and Kosoy2001). Historically, Y. pestis caused pandemics of plague along human migration routes, but today human plague cases occur in localized epidemics described as ‘plague foci’ (Biggins & Kosoy Reference Biggins and Kosoy2001, Malek et al. Reference Malek, Bitam, Levasseur, Terras, Gaudart and Azza2017).
Patterns of plague outbreaks and Y. pestis persistence in host reservoirs are still not fully understood (Gage & Kosoy Reference Gage and Kosoy2005). Malek et al. (Reference Malek, Bitam, Levasseur, Terras, Gaudart and Azza2017), for example, suggested that higher soil salinity may contribute to Y. pestis persistence in soil, and possibly influence the persistence and distribution of plague foci. Some mammals also exhibit resistance to Y. pestis, and they may carry the organism while unaffected or even seroconvert and rid themselves of the bacteria (Biggins & Kosoy Reference Biggins and Kosoy2001). Canids exhibit widespread resistance and rarely die from plague, whereas felids are much more susceptible to Y. pestis and often suffer clinical symptoms of plague, including death (Biggins & Kosoy Reference Biggins and Kosoy2001). This variation in resistance exhibited by different species is likely a factor influencing the distribution of plague foci, as well as the persistence of Y. pestis among some wild animal populations in specific geographical areas (Gage & Kosoy Reference Gage and Kosoy2005).
We know that fossorial rodents exposed to contaminated soil can contract the disease and function as enzootic, primary hosts responsible for maintaining Y. pestis in a focal area (Biggins & Kosoy Reference Biggins and Kosoy2001, Gage & Kosoy Reference Gage and Kosoy2005). Fleas that feed on infected rodents are the principal vectors for transmission to other rodents, as well as to other animals or humans that might spend time near rodent burrows (Salkeld & Stapp Reference Salkeld and Stapp2006). Fleas collected in burrows have tested positive for Y. pestis more than a year after their host animals have died (Gage & Kosoy Reference Gage and Kosoy2005).
Plague epizootics in wildlife generally occur when the pathogen passes from enzootic to epizootic hosts, such as prairie dogs (Cynomys spp.), in which plague spreads rapidly and is more easily detected due to high mortality rates. Carnivores are thought to acquire Y. pestis primarily through predation on rodent hosts, supported by the fact that plague exposure is often detected via serology in smaller carnivores that hunt them. Y. pestis may also be passed up the food chain as larger carnivores kill and consume smaller carnivores. It may also be passed among carnivores indirectly through the transmission of fleas or directly via aerosol, body fluids or fleas during social contact (Salkeld & Stapp Reference Salkeld and Stapp2006). Humans may be exposed by enzootic or epizootic hosts, by carnivores that eat these hosts or by fleas that are carried by any of these animals (Fig. 1) (e.g., Gage et al. Reference Gage, Dennis, Orloski, Ettestad, Brown and Reynolds2000, Wong et al. Reference Wong, Wild, Walburger, Higgins, Callahan and Czarnecki2009).
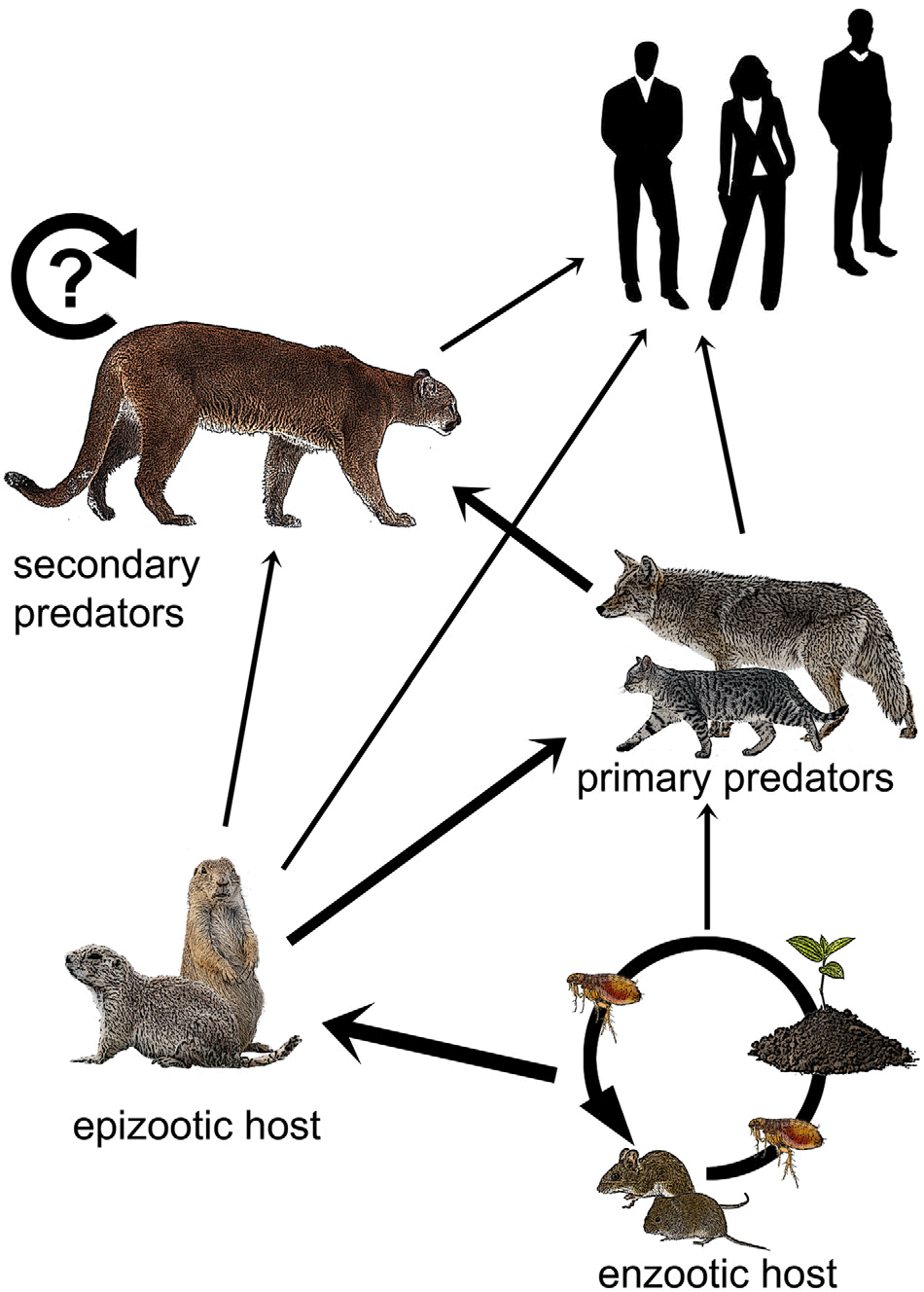
Fig. 1. Hypothetical plague transmission to humans. The wider arrows represent primary modes of transfer and the thinner arrows represent less frequent ones. The circular arrow depicts the potential reservoir in pumas. Enzootic hosts maintain plague foci through interactions with fleas and infected soils. Plague is transferred to epizootic hosts such as prairie dogs and ground squirrels, then up the food chain from smaller carnivores, such as house cats and coyotes, to secondary predators, such as pumas. Pumas may also be primary predators of epizootic hosts. Humans may be exposed through contact with any of these animal groups.
Wild felids have recently been hypothesized as potential reservoirs for Y. pestis, meaning that they might be able to maintain the disease in the absence of an enzootic host (Salkeld & Stapp Reference Salkeld and Stapp2006, Bevins et al. Reference Bevins, Tracey, Franklin, Schmit, MacMillan and Gage2009). Bevins et al. (Reference Bevins, Tracey, Franklin, Schmit, MacMillan and Gage2009) also suggested pumas (Puma concolor) may spread plague, as pumas disperse long distances and could quickly carry plague-infected fleas from place to place (Salkeld & Stapp Reference Salkeld and Stapp2006), reinvigorating old plague foci or establishing new ones. Recent research has highlighted that pumas are more social than previously thought (Elbroch et al. Reference Elbroch, Levy, Lubell, Quigley and Caragiulo2017b), and their close proximity may support the hypothesis that direct host-to-host transmission may occur in this species, either via fleas, body fluids or aerosol.
We sampled for plague in a puma population in the southern Greater Yellowstone Ecosystem (GYE) during the time period and in the exact area when and where a boy became infected with plague in 2008 (Angel Reference Angel2008). We tested two competing hypotheses that might explain patterns of prevalence among pumas: sociality versus age-specific foraging. In support of sociality explaining patterns of prevalence, we made two predictions: (1) males would test positive for exposure to Y. pestis more than females because they interact with other unrelated pumas more often than females (Elbroch et al. Reference Elbroch and Quigley2016); and (2) older pumas would exhibit a higher prevalence of antibodies to Y. pestis than younger pumas, as social interactions accumulate over time. In support of age-specific foraging influencing prevalence, we predicted younger pumas would test positive more often than older pumas because they eat more small prey (Elbroch & Quigley Reference Elbroch and Quigley2019).
Materials and methods
Our study area encompassed c. 2300 km2 in the GYE in southern Teton County, Wyoming, USA. Elevations in the study area range from 1800 m in the valleys to >3600 m in the mountains. The area was characterized by short, cool summers and long, cold winters with frequent snowstorms. Average summer temperatures were 6.9°C and average winter temperatures were –7.2°C (Gros Ventre SNOTEL weather station). Precipitation occurred mostly as snow, and maximum snow depths ranged from 100 cm at lower elevations to >245 cm at intermediate and higher elevations (≥2000 m). Habitats included foothill grasslands, big sagebrush (Artemisia tridentata)-dominated shrub-steppe, aspen (Populus tremuloides) forests and higher-elevation coniferous forests, composed predominantly of Douglas fir (Pseudotsuga menziesii), lodge pole pine (Pinus contorta), subalpine fir (Abies lasiocarpa) and Engelmann spruce (Picea engelmannii).
The area was widely inhabited by Uinta ground squirrels (Urocitellus armatus), a fossorial rodent known to carry plague, as well as coyotes (Canis latrans), American badgers (Taxidea taxus) and other smaller carnivores known to be puma prey and to carry plague (Salkeld & Stapp Reference Salkeld and Stapp2006). The GYE is also inhabited by brown bears (Ursus arctos), American black bears (Ursus americanus), wolves (Canis lupus), coyotes, red foxes (Vulpes vulpes), elk (Cervus canadensis), mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), moose (Alces alces), bighorn sheep (Ovis canadensis), North American pronghorn (Antilocapra americana) and numerous smaller mammals.
We tested for Y. pestis exposure in pumas from 2005 to 2014 as part of routine puma captures. We estimated puma age in months using tooth condition (Heffelfinger Reference Heffelfinger2010) and gum line recession (Laundré et al. Reference Laundré, Hernández, Streubel, Altendorf and González2000), and we recorded gender, weight and standardized body measurements. We fitted pumas with telemetry collars and collected blood for disease and genetic analyses. Blood sera underwent complement-enzyme-linked immunosorbent assay (cELISA) analyses following protocols established in Chu (Reference Chu2000), with sensitivity/specificity set by methods described in Williams et al. (Reference Williams, Arntzen, Robinson, Cavanaugh and Isaacson1982) to detect Y. pestis antibodies. Specifically, laboratory personnel determined plague exposure via an increase in the serum dilution producing an ‘obvious’ reaction from 1:4 to 1:32 or more (≥4 times) (Williams et al. Reference Williams, Arntzen, Robinson, Cavanaugh and Isaacson1982).
All collars were equipped with mortality sensors, which alerted us when an individual had not moved for ≥8 hours. Whenever possible, we determined the cause of death through a combination of interpreting field signs (e.g., bite marks, evidence of fighting, footprints), assessing the external condition of carcasses, X-rays of carcasses removed from the field and laboratory analyses of blood and tissue samples conducted by the Wyoming Game and Fish Wildlife Health Laboratory assessing infections and diseases. All puma carcasses tested for plague described in this paper were necropsied by a veterinarian. During necropsy, two fresh tissue samples (one in a Whirl-Pak sampling bag and one suspended in formalin) were collected for liver, spleen, kidney, heart, lung, axillary lymph nodes and retropharyngeal lymph nodes. Additional samples suspended in formalin included muscle tissue, submandibular lymph tissue, salivary gland, diaphragm muscle, oesophagus, trachea, stomach, small intestine, large intestine and duodenum. We separated the head from the carcass and sent it in its entirety. In the laboratory, additional samples of the third eyelid, tonsil, brain and various lymph nodes were collected and analysed as well. Cultures developed from tissue samples were subjected to polymerase chain reaction tests, direct fluorescent antibody tests and ELISAs in order to directly detect the presence of Y. pestis (Chu Reference Chu2000).
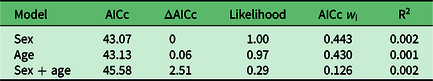
Using logistic regression, we built three simple a priori models (Table 1) to test whether puma age, sex or the combination of the two best fit our binary selection parameter: positive/negative exposure to or detection of Y. pestis. The age of pumas in our analyses reflected the age of each individual (in months) at which we first detected plague or antibodies to Y. pestis, or in negative animals the oldest age at which they were sampled. We calculated the Akaike information criterion adjusted for small sample size (AICc), ΔAICc and Akaike weights (w i) for each model (Burnham & Anderson Reference Burnham and Anderson2002). We evaluated the fit of the best model(s) with a modified R2 calculation appropriate for logistic regression: –L(model)/–L(reduced), where L is the log likelihood.
Table 1. Results of serum tests for Yersinia pestis antibodies among pumas sampled from 2005 to 2014 in northwest Wyoming.

cELISA = complement-enzyme-linked immunosorbent assay.
Results
Of 28 pumas tested for Y. pestis antibodies or the presence of the bacteria in tissues, 12 tested positive (42.9%). Antibodies to Y. pestis were detected in 8 of 17 (47%) pumas tested by cELISA (Table 1), and the organism itself was detected in 4 of 11 (36%) pumas tested after necropsy (Table 2). All four of the pumas that were positive at necropsy died from the disease, as well as exhibited symptoms of pneumonia. One puma (M21) tested negative by serology twice over a 3-year period, then tested positive 1 year after the second negative test, and then negative again 1 year later. This sequence of tests over a 5-year period suggests that this animal was exposed at a discrete point in time, developed antibodies and then overcame the bacteria and became negative for antibodies again. In another case, another puma that appeared healthy tested positive for antibodies by serology twice 3 years apart, suggesting possible multiple exposures over time with no apparent disease symptom development.
Table 2. Presence/absence results for Yersinia pestis in the tissues of pumas tested from 2005 to 2014 in northwest Wyoming.
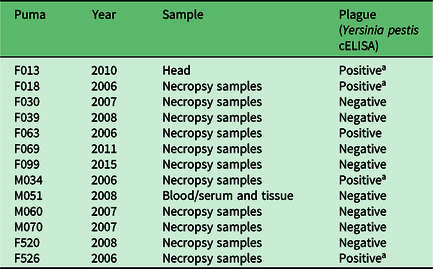
a Died of plague.
cELISA = complement-enzyme-linked immunosorbent assay.
All three of the models we tested to explore patterns of Y. pestis exposure in our study population performed poorly (Table 3). Neither sex nor age nor the combination of the two significantly explained patterns of Y. pestis antibody or disease prevalence among our research animals; this interpretation may have been an artefact of sample size.
Table 3. Model comparison, associated Akaike information criterion adjusted for small sample size (AICc) metrics and R2 to reflect model fit.
Discussion
Here, we report plague from the ecologically important GYE, among the largest remaining ecosystems hosting a suite of large carnivores and migratory ungulates in North America. A total of 43% of pumas sampled tested positive for plague exposure or disease, suggesting a similar chance of exposure for pumas, and possibly humans, to that found in recognized plague-endemic areas, such as the western slope of Colorado (Bevins et al. Reference Bevins, Tracey, Franklin, Schmit, MacMillan and Gage2009). Surprisingly, we documented more evidence of exposure than in other known plague foci, such as southern California and the front range of Colorado (Bevins et al. Reference Bevins, Tracey, Franklin, Schmit, MacMillan and Gage2009), both of which are predicted to be high-risk areas of potential zoonotic transmission (Walsh & Haseeb Reference Walsh and Haseeb2015).
Four pumas died of plague, representing 6.6% of sub-adult and adult mortality reported in Elbroch et al. (Reference Elbroch, Marescot, Quigley, Craighead and Wittmer2018b). Three of the four puma plague mortalities occurred in 2006, 2 years prior to a human plague fatality in the area (Angel Reference Angel2008). Pumas, therefore, may prove to be a sentinel for potential plague risk to humans in western North America, or in refining maps depicting risk of zoonotic transmission (Walsh & Haseeb Reference Walsh and Haseeb2015). Across the western USA and Canada, >3000 pumas are hunted legally each year and presented to state and provincial wildlife agencies, providing opportunities for tissue sampling to test for Y. pestis in conjunction with location data. Our results also highlight the need for education regarding the potential transmission of plague from pumas to humans among hunters, trappers, taxidermists, biologists and managers that handle live and dead pumas. For example in 2007, a puma researcher contracted plague in Arizona during a routine necropsy and died of pneumonic plague, a case that could have been averted with antibiotics had awareness of the possibility been more widespread (Wong et al. Reference Wong, Wild, Walburger, Higgins, Callahan and Czarnecki2009).
Plague and other infectious diseases are expected to increase the costs of social behaviours (Biggins & Kosoy Reference Biggins and Kosoy2001); however, our results did not support this hypothesis. Our sample was small, but neither puma age nor sex explained the patterns of plague prevalence among our study animals. It is tempting to interpret these results as evidence that the benefits of social interactions among pumas outweigh the potential costs (sensu Elbroch et al. Reference Elbroch, Levy, Lubell, Quigley and Caragiulo2017b), but our research lacked the data to test this hypothesis directly. With regards to the influence of puma age on plague patterns, the sequential sera results exhibited by M21, which were negative then positive and then negative for antibodies, also revealed a flaw in our hypotheses. Puma age may not explain the patterns in the detection of antibodies if older animals seroconvert and do not retain detectable antibodies.
Finally, our failure to reject the hypotheses regarding the influence of puma age and sex on plague patterns suggests pumas are not reservoirs for Y. pestis. Plague, however, did kill a 3-month-old kitten that likely contracted the disease from her mother in social contact in the den or via shared fleas (Salkeld & Stapp Reference Salkeld and Stapp2006). In contrast to supporting our hypotheses, our results regarding puma age and sex may in fact support the current hypothesis that apex predators acquire Y. pestis via the consumption of infected prey (Salkeld & Stapp Reference Salkeld and Stapp2006).
We did not, however, find support for our hypothesis that age-specific foraging would explain the patterns of infection. Nevertheless, we suggest future research continues to test whether diet explains patterns of plague among pumas, as we lacked suitable prey selection data across all pumas and all years of our study to conduct these analyses. There is new evidence that pumas exhibit intraspecific differences in diet due to age and behavioural stage (e.g., transient versus resident) (Elbroch et al. Reference Elbroch, Feltner and Quigley2017a, Elbroch & Quigley Reference Elbroch and Quigley2019). When programmed to acquire frequent fixes, new GPS collars also increase opportunities for researchers to conduct site investigations in order to document small puma prey (Elbroch et al. Reference Elbroch, Lowrey and Wittmer2018a). Using this technology, future research could more specifically assess the role of eating small mammals, including carnivores such as coyotes, house cats and badgers (Gage et al. Reference Gage, Dennis, Orloski, Ettestad, Brown and Reynolds2000, Salkeld & Stapp Reference Salkeld and Stapp2006), in plague and other disease patterns among pumas and other apex predators.
Acknowledgements
We thank the Wyoming Game and Fish Wildlife Health Laboratory for complimentary plague analyses and B Smith, S Smith and other members of our field team for their contributions. We thank S Riley, N Polunin and several anonymous reviewers for their constructive comments on earlier versions of the manuscript.
Financial support
We thank the Summerlee Foundation, Charles Engelhard Foundation, Eugene V & Clare E Thaw Charitable Trust, National Geographic Society, Connemara Fund, Tapeats Foundation, the Community Foundation of Jackson Hole, the Lee and Juliet Folger Fund, L Westbrook, the Scully Family, the Haberfelds, the Holders, S and L Robertson, B and L Heskett, F and B Burgess, J Morgan, A Smith, D Bainbridge, T Thomas and numerous other donors too numerous to list.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with applicable national and institutional ethical guidelines on the care and use of laboratory or otherwise regulated animals (Jackson Institutional Animal Care and Use Committee Protocol 027-10EGDBS-060210).






