Published online by Cambridge University Press: 28 February 2024
The Sinya Beds of the Amboseli Basin in Tanzania and Kenya consist largely of carbonate rocks and Mg-rich clays that are intensely deformed where exposed in and near former meerschaum mines. The carbonate rocks consist of limestone and dolomite in Tanzania, but only dolomite has been identified in Kenya. Sepiolite and mixed-layered kerolite/stevensite (Ke/St) are subordinate constituents of the carbonate rocks. The carbonate rocks and overlying bedded sepiolite were deposited in a semiarid lake basin at the foot of the large volcano Kilimanjaro. Calcite and dolomite of the carbonate rocks have δ18O values 4–6‰ lower than calcite and dolomite of the late Pleistocene Amboseli Clays, suggesting that the Sinya Beds were deposited in the middle or early Pleistocene under a different climatic regime when meteoric water had lower δ18O values than at present.
Mg-rich clay minerals form veins and fill cavities in the Sinya Beds. The principal clay minerals are sepiolite and Ke/St, some of which contains substantial Al and Fe (Al-Ke/St). NEWMOD® modeling and other X-ray diffraction (XRD) data suggest that most of the Ke/St contains 25–50 percent kerolite layers, but minor amounts of kerolite-rich Ke/St are present in some samples. Illite with an inferred high content of Fe or Mg is a minor constituent of the samples with Al-Ke/St. The cavity-filling clays were chemically precipitated, as shown by field relationships and SEM study. The early-deposited clays of veins and cavities are principally Ke/St with minor sepiolite, and the latest clay is sepiolite (meerschaum), generally with minor Ke/St.
The δ18O values of cavity-filling Ke/St range from 22.5–25.6‰ and correlate with mineral composition, with the highest values associated with the highest content of stevensite and the lowest values with the highest content of kerolite. This relation suggests that high salinities favored stevensite and low salinities favored kerolite. δ18O values of sepiolite (meerschaum) fall in the middle of the range for Ke/St, suggesting that salinity was not the main control on sepiolite precipitation. High values of aSiO2/aMg2+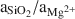 may have been a major factor in sepiolite precipitation.
may have been a major factor in sepiolite precipitation.
Different mixtures of dilute ground water and saline, alkaline lake water in pore fluids may largely account for the differences in clay mineralogy of cavity-filling clays. Sepiolite is the dominant clay mineral in lacustrine sediments of the Amboseli Basin, and the cavity-filling sepiolite may reflect a high proportion of lake water. The low-Al Ke/St may have formed from fluids with a higher proportion of ground water. Detrital clay was very likely a factor in forming the Al-Ke/St, for which δ18O values suggest a saline environment.