Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Grasso, D.
Smets, B. F.
Strevett, K. A.
Machinist, B. D.
van Oss, C. J.
Giese, R. F.
and
Wu, W.
1996.
Impact of Physiological State on Surface Thermodynamics and Adhesion of Pseudomonas aeruginosa.
Environmental Science & Technology,
Vol. 30,
Issue. 12,
p.
3604.
Carew, Evelyn Owen
Cooke, Francis W.
Lemons, Jack E.
Ratner, Buddy D.
Vesely, Ivan
and
Vogler, Erwin
1996.
Biomaterials Science.
p.
23.
Li, Z.
Giese, R. F.
Wu, W.
Sheridan, M. F.
and
van Oss, C. J.
1997.
THE SURFACE THERMODYNAMIC PROPERTIES OF SOME VOLCANIC ASH COLLOIDS.
Journal of Dispersion Science and Technology,
Vol. 18,
Issue. 3,
p.
223.
Khayat, C.
Vatelot, A.
Decloux, M.
and
Bellon-Fontaine, M.N.
1997.
Evaluation of physico-chemical interactions in cross-flow filtration in the particular case of mineral membranes and sugar remelts.
Journal of Membrane Science,
Vol. 137,
Issue. 1-2,
p.
219.
van Oss, Carel Jan
1997.
Hydrophobicity and hydrophilicity of biosurfaces.
Current Opinion in Colloid & Interface Science,
Vol. 2,
Issue. 5,
p.
503.
Azeredo, Joana
Ramos, Ilia
Rodriguks, Ligia
Oliveira, Rosrio
and
Teixeira, Jos
1997.
YEAST FLOCCULATION: A NEW METHOD FOR CHARACTERISING CELL SURFACE INTERACTIONS.
Journal of the Institute of Brewing,
Vol. 103,
Issue. 6,
p.
359.
Liu, Yue
and
Nancollas, George H.
1997.
Crystallization and Colloidal Stability of Calcium Phosphate Phases.
The Journal of Physical Chemistry B,
Vol. 101,
Issue. 18,
p.
3464.
Van Oss, C. J.
Giese, R. F.
and
Wu, W.
1997.
On the Predominant Electron-Donicity of Polar Solid Surfaces.
The Journal of Adhesion,
Vol. 63,
Issue. 1-3,
p.
71.
Naim, J.O
van Oss, C.J
Ippolito, K.M.L
Zhang, J.-W
Jin, L.-P
Fortuna, R
and
Buehner, N.A
1998.
In vitro activation of human monocytes by silicones.
Colloids and Surfaces B: Biointerfaces,
Vol. 11,
Issue. 1-2,
p.
79.
Dourado, F.
Gama, F.M.
Chibowski, E.
and
Mota, M.
1998.
Characterization of cellulose surface free energy.
Journal of Adhesion Science and Technology,
Vol. 12,
Issue. 10,
p.
1081.
Wu, Ŵeénju
and
Nancollas, George H.
1998.
THE FLOCCULATION OF AQUEOUS SUSPENSIONS OF TITANIA INDUCED BY SURFACE NUCLEATION OF CALCIUM PHOSPHATES#.
Journal of Dispersion Science and Technology,
Vol. 19,
Issue. 6-7,
p.
761.
van Oss, Carel J.
1998.
CURRICULUM VTTAE.
Journal of Dispersion Science and Technology,
Vol. 19,
Issue. 6-7,
p.
699.
Bardot, F.
Villiéras, F.
Michot, L.J.
Francois, M.
Gérard, G.
and
Cases, J.M.
1998.
HIGH RESOLUTION GAS ADSORPTION STUDY ON ILLITES PERMUTED WITH VARIOUS CATIONS ASSESSMENT OF SURFACE ENERGETIC PROPERTIES.
Journal of Dispersion Science and Technology,
Vol. 19,
Issue. 6-7,
p.
739.
Good, Robert J.
Islam, Mojahedul
Baier, Robert E.
and
Meyer, Anne E.
1998.
THE EFFECT OF SURFACE HYDROGEN BONDING (ACID-BASE INTERACTION) ON THE HYDROPHOBICITY AND HYDROPHILICITY OF COPOLYMERS: VARIATION OF CONTACT ANGLES AND CELL ADHESION AND GROWTH WITH COMPOSITION.
Journal of Dispersion Science and Technology,
Vol. 19,
Issue. 6-7,
p.
1163.
Jin, Yu-Lai
and
Alex Speers, R.
1998.
Flocculation of Saccharomyces cerevisiae.
Food Research International,
Vol. 31,
Issue. 6-7,
p.
421.
Wiegel, D
Kaufmann, J
and
Arnold, K
1999.
Polar interactions of chondroitinsulfate.
Colloids and Surfaces B: Biointerfaces,
Vol. 13,
Issue. 3,
p.
143.
Teixeira, P.
and
Oliveira, R.
1999.
Influence of surface characteristics on the adhesion of Alcaligenes denitrificans to polymeric substrates.
Journal of Adhesion Science and Technology,
Vol. 13,
Issue. 11,
p.
1287.
NANCOLLAS, GEORGE H.
and
WU, WENJU
1999.
CRYSTAL GROWTH AND DISSOLUTION OF CALCIUM PHOSPHATES: A KINETICS AND SURFACE ENERGY APPROACH.
Phosphorus Research Bulletin,
Vol. 10,
Issue. 0,
p.
226.
Vogler, Erwin A.
1999.
Water and the acute biological response to surfaces.
Journal of Biomaterials Science, Polymer Edition,
Vol. 10,
Issue. 10,
p.
1015.
Hermansson, Malte
1999.
The DLVO theory in microbial adhesion.
Colloids and Surfaces B: Biointerfaces,
Vol. 14,
Issue. 1-4,
p.
105.
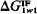 exactly zero. When
exactly zero. When 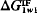 is positive, the interaction of the material with water dominates and the surface of the material is hydrophilic; when
is positive, the interaction of the material with water dominates and the surface of the material is hydrophilic; when 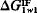 is negative, the polar cohesive attraction between the water molecules dominates and the material is hydrophobic. Thus, the sign of
is negative, the polar cohesive attraction between the water molecules dominates and the material is hydrophobic. Thus, the sign of 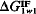 defines the nature of the surface and the magnitude of
defines the nature of the surface and the magnitude of 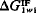 may be used as the natural quantitative measure of the surface hydrophobicity or hydrophilicity.
may be used as the natural quantitative measure of the surface hydrophobicity or hydrophilicity.