Maternal feeding during pregnancy is considered an important factor for maternal–fetal health(Reference Ramakrishnan, Grant and Goldenberg1). Dietary inadequacy during this period may be a risk factor for excessive weight gain(Reference Shin, Lee and Song2) and adverse gestational outcomes(Reference Silva-Zolezzi, Samuel and Spieldenner3), in addition to repercussions for fetal nutritional status(Reference Shapiro, Kaar and Crume4) and the future health of the child(Reference Zhu, Olsen and Mendola5).
Recent studies have suggested that not only what and how much we eat but also when we eat have a significant effect on energy balance(Reference de Castro6), weight regulation(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar7–Reference McHill, Phillips and Czeisler9) and glycaemic control(Reference Jakubowicz, Barnea and Wainstein8,Reference Leung, Huggins and Bonham10) . However, the timing of food intake is not well studied among pregnant women, who are considered an at-risk group in terms of nutritional health. Only a few studies with pregnant women conducted in this field have shown that meal timing during pregnancy is an important factor in determining maternal glycaemic levels(Reference Chandler-Laney, Schneider and Gower11–Reference Loy, Chan and Wee13), offspring birth size and adiposity(Reference Loy, Wee and Colega14).
Meal timing seems to influence eating patterns, diet quality(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar7,Reference Gontijo, Cabral and Balieiro15) and weight regulation(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar7,Reference Aljuraiban, Chan and Oude Griep16) . A study of an adult population showed that higher energy intake in the evening compared with the morning meals seems to influence the increase in BMI(Reference Aljuraiban, Chan and Oude Griep16). A longitudinal study performed with obese and overweight non-pregnant Spanish individuals during 20 weeks of weight loss treatment showed that later eaters of the main meal, lunch in this case, had different energy intake distribution throughout the day, with less energy consumption during breakfast. They also frequently skipped breakfast, had worse weight regulation and had an evening tendency to perform activities(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar7). In this sense, studies have also shown that the timing of eating episodes has a strong association with chronotype, which is defined as the preference to perform activities at certain times of the day(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar7,Reference Nimitphong, Siwasaranond and Saetung17) . A recent study published by our group found that earlier times for the first meal, a higher number of eating episodes, longer eating duration and a morning chronotype tendency, which has been extensively associated with early eating habits(Reference Sato-Mito, Shibata and Sasaki18), were associated with better diet quality in the first gestational trimester(Reference Gontijo, Cabral and Balieiro15).
To date, the influence that the timing of food intake has on eating patterns, diet quality and weight gain is still poorly evaluated in the literature and, to the best of our knowledge, has not been evaluated during gestational trimesters. Therefore, the aim of the present study was to analyse the effect that the timing of food intake has on eating patterns, diet quality and weight gain during pregnancy. We hypothesised that pregnant women with later first and last eating episodes have an inadequate distribution of energy intake throughout the day, that is, with a lower food intake in the morning meals and a greater intake in the evening meals, as well as a poorer diet quality and greater weight gain during pregnancy.
Materials and methods
Design and ethics
A prospective cohort study was carried out with healthy low-risk pregnant women. These pregnant women were attending the antenatal clinics of the public health service in the city of Uberlandia, Minas Gerais, Brazil, between October 2015 and February 2017. The present study was conducted according to the guidelines in the Declaration of Helsinki, and ethical approval was obtained from the Human Research Ethics Committee (protocol number 1.199.829/2015) at the Federal University of Uberlandia. Informed written consent was obtained from all women.
Sample
The study included pregnant women, aged 18 years and older, with no shift workers, all carrying a single fetus and who had undergone their first antenatal visit before the 12th week of pregnancy. Pregnant women with fetal malformation or anomalies, or who tested positive for HIV, syphilis, toxoplasmosis, rubella, cytomegalovirus and varicella, were excluded from the study.
The sample size required for the present study was determined using the G*Power software version 3.1(Reference Faul, Erdfelder and Lang19). The sample size calculations were based on repeated-measures ANOVA, within–between interaction, with an effect size of 0·25, an α level of 0·05, 95 % power, four groups, three measurements, a correlation between repeated measures of 0·5 and a non-sphericity correction ϵ of 1. Given these specifications, a total sample of sixty women was required at the final follow-up. Considering a 20 % adjustment for possible losses, a minimum of seventy-two women was needed at baseline.
During the period of the study, 142 women in the first trimester of pregnancy were invited to participate. Eleven pregnant women refused to participate, ten were excluded because they did not meet the age criteria and twenty-one had not completed all of the evaluations. A final sample of 100 pregnant participants was used in the study.
Data collection
The data collection occurred at three time points – once per trimester: first trimester: 4th–12th gestational week; second: 20th–26th week and third: 30th–37th week. Interviews and measurements were conducted by trained researchers, while the pregnant women were waiting for medical appointments in the public service units.
Initial questionnaire
A structured questionnaire enquiring about marital status, level of education, clinical history and physical activity was used. In the first trimester, the women were asked about their marital status (married/living with a partner or single), and education was recorded by asking women about their highest level of education. In each trimester, we asked whether the pregnant woman had experienced any episodes of nausea in the last 30 d and the frequency of these episodes. Physical activity was assessed using a questionnaire in which women reported if they had performed any physical activity during the last month in each trimester (yes/no) and the type, frequency and duration of this physical activity.
Chronotype
To determine chronotype, the participants were asked to report their usual bedtime, wake-up time, sleep-onset latency and sleep duration on weekdays and weekends during each trimester. Chronotype was derived using mid-sleep time on free days (MSF), with a further correction for calculated sleep debt, and calculated as the difference between average sleep duration at weekends and on weekdays(Reference Roenneberg, Kuehnle and Juda20). Higher coefficients indicate an evening preference, while lower coefficients indicate morning preference.
Anthropometric measurement and weight gain assessment
Height was measured in the first trimester with a stadiometer fixed to the wall, with an accuracy of 0·1 cm (Welmy®). The pre-pregnancy weight was self-reported, and the weight at each trimester was measured with a set of scales to an accuracy of 0·1 kg (Welmy®). BMI was calculated as weight (kg) divided by height squared (m2). To determine pre-pregnancy BMI, we used the classifications of the World Health Organization(21).
Weight gain was calculated in each gestational trimester using the current measured weight value subtracted from the value of the weight evaluated in the previous trimester or pre-gestational weight in the case of the first trimester. In each trimester, all pregnant women were not evaluated in the same gestational week; therefore, the value of weight gain was divided by the gestational week of the evaluation to obtain the value of the weight gain per week. The value of the weight gain per week was used to evaluate the weight gain during pregnancy. Pregnant women who lost weight were excluded from the analysis in the gestational trimesters that presented this loss, since the goal was to evaluate the weight gain. Therefore, a total of nine pregnant women in the first and second trimesters and six in the third trimester were excluded.
Dietary assessment
For each trimester, the information regarding dietary intake was assessed by three 24-h dietary recalls (24HR) using the five-stage multiple-pass interviewing technique(Reference Conway, Ingwersen and Vinyard22), conducted by trained researchers. This required women to report an uninterrupted list of all foods and beverages consumed, answer a forgotten food list, provide details about times, meals and snacks names and a description of foods, including brand names and recipes for home-cooked meals, as well as amounts eaten. The interview ended with a final probe review. Portion sizes were estimated using food pictures of various portion sizes and common household measurements such as cups, glasses, bowls, teaspoons and tablespoons, in addition to individual food items/units.
The first 24HR was collected at the time of the interview, and the other two were conducted through telephone interviews(23). The 24HR were conducted on non-consecutive days, including one at the weekend, and the average consumption during 3 d in each trimester was used for analysis. The 24HR that showed implausible data with energy intakes of less than 2092 kJ/d or more than 14 644 kJ/d(Reference Loy, Wee and Colega14) were excluded and were not included in the calculations of average consumption. In this case, the plausible data of the other 24HR of the participant were included in the analysis.
The software Dietpro®, version 5i, was used to calculate the nutrients of food intake, and a Brazilian database(24) was preferentially used as a reference, followed by nutrient information from food labels and the United States Department of Agriculture international nutrient database(25).
Eating patterns
The distribution of energy and macronutrients throughout the day was evaluated by the percentage of total daily energy intake and the percentage of energy intake from protein, fat and carbohydrates segregated in four mealtimes: morning (breakfast and mid-morning snacks), lunch, afternoon (afternoon snacks) and evening (dinner and night-time snacks). To classify the types of meals or snacks (breakfast, mid-morning snacks, lunch, afternoon snacks, dinner and night-time snacks), we considered participants’ perceptions of the type of meal and/or snacks(Reference Trancoso, Cavalli and Proença26) and also analysed the type of food often consumed by the Brazilian population at every meal(Reference Sato, Fujimori and Szarfarc27).
Time-related eating patterns were evaluated by the number of eating episodes, eating duration and night fasting. The number of eating episodes was determined by the number of energetic events ≥209·2 kJ/d, with time intervals between food and/or beverage consumptions of ≥15 min(Reference Gibney and Wolever28) reported in the 24HR. Eating duration was determined by the length between the first and last energetic event in the 24HR(Reference Gill and Panda29). Night-fasting interval was determined by calculating the longest fasting interval between eating episodes from 19.00 hours to 06.59 hours(Reference Loy, Wee and Colega14). All variables were calculated using the average of the 24HR in each trimester.
Diet quality
Diet quality was assessed using the Brazilian Healthy Eating Index-Revised (BHEI-R)(Reference Previdelli, Andrade and Pires30), validated for the Brazilian population(Reference Andrade, Previdelli and Marchioni31). The BHEI-R is similar to the Healthy Eating Index-2005(Reference Guenther, Reedy and Krebs-Smith32) and is composed of twelve components classified as adequate foods: total fruit, whole fruit, total vegetables, dark green and orange vegetables and legumes, total grains, whole grains, milk and dairy products, meat, eggs and legumes, and oils; and moderate foods: saturated fat, Na and energy content from solid fats, alcoholic beverages and added sugars(Reference Previdelli, Andrade and Pires30). The number of daily servings was adjusted by the energy density (4184 kJ/d). Each component received a specific score, ranging from 0–5, 0–10 or 0–20. The maximum score is given for an intake greater than or equal to the portions recommended and a zero score for no consumption of the components classified as adequate. However, the proportion for the components classified as moderate food is inverse: higher consumption means a lower score. Intermediate values of intake are given proportional scores. The total BHEI-R is the addition of the scores of the components and can reach up to 100 points, with the higher scores indicating a healthy diet quality(Reference Previdelli, Andrade and Pires30).
Timing of food intake
The timing of sunrise and sunset is an important aspect to consider when studying the timing of food intake, especially in countries with seasonal variations(Reference Arendt33). In Uberlandia (18° South, 48° West), a city located southeast of Brazil, sunrise and sunset occurred, respectively, at 06.17 and 18.23 hours on 20 March 2016 (autumnal equinox); at 06.45 and 17.44 hours on 20 June 2016 (winter solstice); at 06.02 and 18.09 hours on 22 September 2016 (vernal equinox) and at 06.33 and 19.49 hours on 21 December 2016 (summer solstice), considering daylight saving time(34). Therefore, sunrise in Uberlandia showed little variation throughout the year and occurred at 06.24 hours on average, while sunset (annual average of 18.31 hours) had a slightly higher variation due to daylight saving time. However, as in the present study, the timing of food intake did not change during pregnancy (online Supplementary Table S1) and the mean of the values reported in the 24HR for the three trimesters was considered the usual mealtime for pregnant women.
The classification of timing of food intake was based on the time of the first concomitant with the last eating episodes: energetic events ≥209.2 kJ/d with time intervals between the consumption of food and/or beverages of ≥15 min. The first eating episode was defined as the first energetic event after waking, and the last eating episode was defined as the last energetic event before bedtime. In addition, for women who woke up in the night to eat, this energetic event was considered the last eating episode and computed as a night-time snack. Based on the usual timing of the first and last eating episodes (mean values), pregnant women were classified as early or late if these values were below or above the median of the population, respectively (median: first eating episode = 08.38 hours; last eating episode = 20.20 hours). Four groups were obtained: Early/Early: early first and last eating episode; Early/Late: early first and late last eating episode; Late/Early: late first and early last eating episode and Late/Late: late first and last eating episode.
Statistical analyses
Statistical analyses were performed according to the classification of the timing of the first and last eating episodes. The normality of the data was established using Kolmogorov–Smirnov tests. Categorical data were shown as frequencies and percentages, while continuous data were shown as means and standard deviations or medians and interquartile ranges. Descriptive analyses of maternal characteristics between the groups were performed using ANOVA or Kruskal–Wallis tests (continuous variables), and Fisher’s exact test (categorical variables). Generalised estimating equation models were used to determine the effects of timing (early or late) of the first and last eating episodes and gestational trimesters (independent variables) on eating patterns (percentage of the total daily energy and macronutrients intakes in the meals, number of eating episodes, eating duration and night fasting), energy and macronutrients total daily intakes, diet quality (scores of the total BHEI-R and its components), BMI and weight gain during pregnancy (dependent variables). Gamma distributions were used, and multiple comparisons were performed using the Bonferroni post hoc test when necessary. All models were adjusted for age, pre-gestational BMI, schooling, chronotype (MSF), physical activity (yes/no) and frequency of nausea in the last 30 d; with an exception for BMI during pregnancy, the adjustment for pre-gestational BMI was not used. Statistical analyses were performed using SPSS 20.0, and a P-value of <0·05 was considered statistically significant.
Results
Maternal characteristics
Maternal characteristics according to the groups are presented in Table 1. The pregnant women in the Early/Early group had a higher median age compared with the Late/Early group. There was also a significant difference between the groups in terms of chronotype: the Late/Early and Late/Late groups had a greater evening preference when compared with the Early/Early and Early/Late groups. For the other variables analysed, no differences were found between the groups (Table 1).
Table 1. Demographic, anthropometric, chronobiological and clinical data of women during pregnancy (n 100/each trimester)*
(Mean values and standard deviations; median values and interquartile ranges; numbers and percentages)

a,b Values within a row with unlike superscript letters were significantly different (P < 0·05; Tukey or Dunn post hoc test).
* Early/Early: early first and last eating episodes; Early/Late: early first and late last eating episodes; Late/Early: late first and early last eating episodes; Late/Late: late first and last eating episodes. ANOVA or Kruskal–Wallis, and Fisher’s exact tests.
† Chronotype was derived using mid-sleep time on free days (MSF), with further correction for calculated sleep debt – the difference between average sleep duration at weekends and on weekdays.
The meal and snack times and time-related eating patterns did not change during pregnancy (online Supplementary Table S1). For this reason, the values were presented in the total gestational data values, representing the average of the three trimesters, and the meal and snack times and time-related eating patterns differed according to the defined groups separated by the timing of food intake (Table 2). The Late/Early and Late/Late groups presented a higher prevalence of skipping breakfast and morning snacks, while the Early/Early and Late/Early groups present a higher prevalence of skipping night-time snacks during pregnancy (Table 2). In addition, the Early/Late group presented a higher number of eating episodes when compared with the Late/Early and Late/Late groups. The Early/Late group also presented a higher eating duration than the Early/Early, Late/Early and Late/Late groups. The Early/Early and Late/Late groups presented a greater eating duration than the pregnant women in the Late/Early group. Also, the Early/Early and Late/Early groups presented longer night-fasting intervals when compared with the Early/Late and Late/Late groups (Table 2).
Table 2. Effect of timing of the first and last eating episodes (groups) on meal and snack times and time-related eating patterns during pregnancy (total gestational data values represent the average of the three trimesters, n 100/each trimester)*
(Mean values with their standard errors; numbers and percentages)
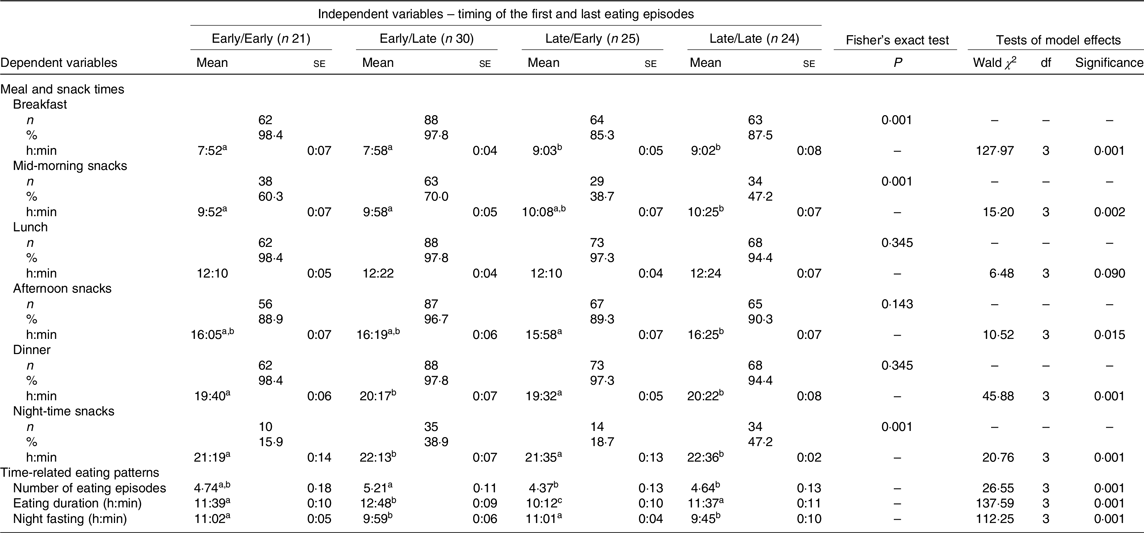
a,b,c Mean values within a row with unlike superscript letters were significantly different in pairwise comparisons (P < 0·05; Bonferroni post hoc test).
* Early/Early: early first and last eating episodes; Early/Late: early first and late last eating episodes; Late/Early: late first and early last eating episodes; Late/Late: late first and last eating episodes. Fisher’s exact test. Generalised estimating equations models, adjusted: age, pre-gestational BMI, schooling, chronotype (MSF: mid-sleep time on free days), physical activity and frequency of nausea in the last 30 d.
Effect of gestational trimester (time) on eating patterns, diet quality, weight gain and BMI during pregnancy
The effect of time (main effect of gestational trimester) was evaluated using the total of pregnant women, without considering the defined groups separated by the timing of food intake. Pregnant women maintained the number of eating episodes, eating duration, night-fasting interval (online Supplementary Table S1) and total energy (kcal) and macronutrient (g) intake during pregnancy (online Supplementary Table S2). They also maintained the distribution of energy and macronutrients in the meals during pregnancy (online Supplementary Table S2).
In relation to diet quality, the pregnant women showed a decrease from the first to the second trimester in terms of the total fruit (3·15 (se 0·17) to 2·57 (se 0·17); P = 0·043) and whole fruit (3·45 (se 0·18) to 2·70 (se 0·20); P = 0·011) components but did not differ from the third trimester (total fruit 2·95 (se 0·17); whole fruit 3·04 (se 0·20)) (online Supplementary Table S3).
As expected for pregnancy, BMI increased over the trimesters (third trimester 29·01 (se 0·43) kg/m2 > second trimester 26·64 (se 0·44) kg/m2 > first trimester 24·76 (se 0·43) kg/m2; P = 0·001) (online Supplementary Table S2). There was also an effect of gestational trimester (time) on weight gain. The weight gain per week increased over the trimesters (third trimester 1·19 (se 0·08) kg/week > second trimester 0·57 (se 0·04) kg/week > first trimester 0·24 (se 0·03) kg/week; P = 0·001) (online Supplementary Table S2).
Effect of timing of the first and last eating episodes (groups) and gestational trimester (time) on eating patterns, diet quality, weight gain and BMI during pregnancy
There was no interaction between the defined groups and trimester (time) on diet quality (online Supplementary Table S4), total energy (kcal) and macronutrient (g) intake, weight gain per week and BMI (Table 3). The results showed that the interaction between groups and trimester (time) only had an effect on percentage of fat intake in the morning eating episodes in the first trimester (Early/Early group 8·20 (se 0·93) % total energy intake (TEI) > Late/Late groups 4·16 (se 0·62) % TEI; P = 0·001) (online Supplementary Table S5).
Table 3. Effect of timing of the first and last eating episodes (groups) and gestational trimesters (time) on total energy and macronutrients intakes, scores of the total Brazilian Healthy Eating Index-Revised (BHEI-R), current BMI and weight gain during the pregnancy (n 100/each trimester)*
(Mean values with their standard errors)

a,b Mean values within a row with unlike superscript letters were significantly different in pairwise comparisons (P < 0·05; Bonferroni post hoc test).
* Early/Early: early first and last eating episodes; Early/Late: early first and late last eating episodes; Late/Early: late first and early last eating episodes; Late/Late: late first and last eating episodes. Generalised estimating equations model, adjusted: age, pre-gestational BMI, schooling, chronotype (MSF: mid-sleep time on free days), physical activity and frequency of nausea in the last 30 d. Weight gain: pregnant women who lost weight were excluded from the analysis, and number of pregnant women who were excluded from these analysis – n (%): first trimester = Early/Early: 2 (9·5); Early/Late: 4 (13·3); Late/Early: 1 (4); Late/Late: 2 (8·3); second trimester = Early/Early: 3 (14·3); Early/Late: 3 (10); Late/Early:0 (0); Late/Late: 3 (12·5); third trimester = Early/Early: 2 (9·5); Early/Late: 2 (6·7); Late/Early: 0 (0); Late/Late: 2 (8·3).
† To convert kcal to kJ, multiply by 4·184.
‡ Total gestational data values represent the average of the three trimesters (n 300; Early/Early: 63; Early/Late: 90; Late/Early: 75; Late/Late: 72).
Effect of timing of the first and last eating episodes (groups) on eating patterns, diet quality, weight gain and BMI
The effect of groups was evaluated using the total gestational data, representing the average of the three trimesters. With regard to total energy consumption and macronutrients, the Early/Late group consumed more total energy (kcal), fat (g) and carbohydrate (g) than the Early/Early and Late/Early groups and more protein (g) than the Late/Early group (Table 3).
Also, the early eaters of the first eating episode consumed a higher percentage of energy (Early/Late group > Late/Late group) and carbohydrate (Early/Late group > Late/Late group) during morning meals, and a lower percentage of energy (Early/Early group < Late/Early and Late/Late groups; Early/Late group < Late/Late group) and carbohydrate (Early/Early group < Late/Early group; Early/Late group < Late/Early and Late/Late groups) during the evening meals (Fig. 1).
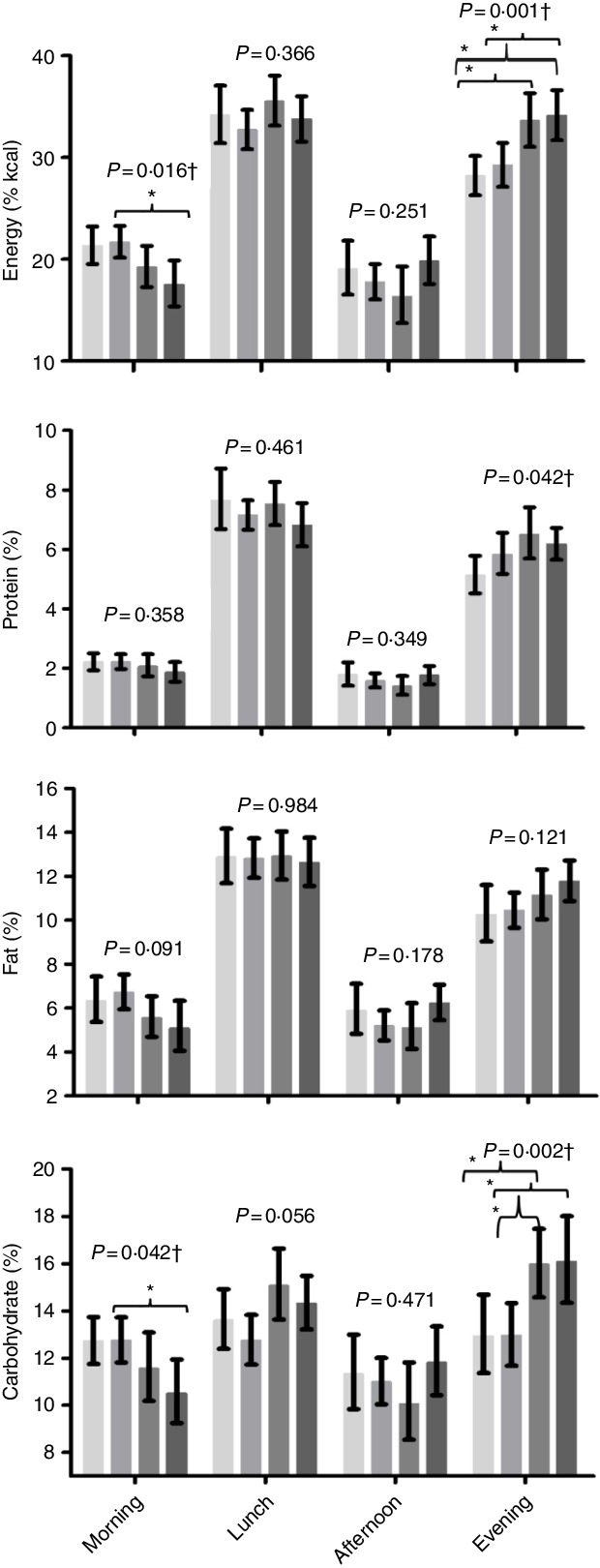
Fig. 1. Effect of timing of the first and last eating episodes (groups) on distribution of energy and macronutrients throughout the day (total gestational data values represent the average of the three trimesters, n 100/each trimester). Note: EE: Early/Early, early first and last eating episodes; EL: Early/Late, early first and late last eating episodes; LE: Late/Early, late first and early last eating episodes; LL: Late/Late, late first and last eating episodes. Generalised estimating equations model, adjusted: age, pre-gestational BMI, schooling, chronotype (MSF: mid-sleep time on free days), physical activity and frequency of nausea in the last 30 d. † Significant tests of model effects. * Bonferroni post hoc test, pairwise comparisons, P < 0·05. Number of pregnant women who had a meal n (%): morning: EE = 61 (96·83); EL = 84 (96·55); LE = 65 (86·67); LL = 67 (89·33); lunch: EE = 62 (98·41); EL = 85 (97·70); LE = 73 (97·33); LL = 71 (94·67); afternoon: EE = 56 (88·89); EL = 83 (95·40); LE = 67 (89·33); LL = 69 (92); night: EE = 60 (95·24); EL = 84 (96·55); LE = 74 (98·67); LL = 74 (98·67).  , EE;
, EE;  , EL;
, EL;  , LE;
, LE;  , LL.
, LL.
The results showed that the defined groups had an effect on diet quality. The early eaters of the first eating episode showed a better diet quality in terms of total fruit (Early/Late group > Late/Early group) and whole fruit components (Early/Late group > Late/Early and Late/Late groups) (Table 4). Our results did not show any effect of groups on BMI and weight gain per week (Table 3).
Table 4. Effect of timing of the first and last eating episodes (groups) on scores of the total Brazilian Healthy Eating Index-Revised (BHEI-R) and its components during the pregnancy (total gestational data values represent the average of the three trimesters, n 100/each trimester)
(Mean values with their standard errors)

Early/Early, early first and last eating episodes; Early/Late, early first and late last eating episodes; Late/Early, late first and early last eating episodes; Late/Late, late first and last eating episodes; SoFAAS, solid fats, alcoholic beverages, and added sugars.
a,b Mean values within a row with unlike superscript letters were significantly different in pairwise comparisons (P < 0·05; Bonferroni post hoc test).
* All fruit including fruits and fruit juice.
† All fruit excluding fruit juice.
‡ Legumes counted as vegetables only after meat, eggs and legumes standard is met.
§ Total grains: cereals, roots and tubers.
‖ Includes milk and other dairy products and soya-based beverages.
¶ Includes monounsaturated and polyunsaturated fats, oils from oilseeds and fat in fish. Generalised estimating equations model, adjusted: age, pre-gestational BMI, schooling, chronotype (MSF: mid-sleep time on free days), physical activity and frequency of nausea in the last 30 d.
Discussion
To the best of our knowledge, this is the first cohort study to investigate the effect that the timing of the first and last eating episode has on eating patterns, diet quality and weight gain in pregnant women. The main finding of our study was that early eaters of the first eating episode consumed a higher percentage of energy and carbohydrate during morning meals and a lower percentage of energy and carbohydrate at evening meals, compared with the late eaters of the first eating episode. The early eaters of the first eating episode showed higher scores for the total fruit and whole fruit components, obtained by BHEI-R. Our results also showed that the early eaters of the first eating episode skipped breakfast less often and consumed more eating episodes per d. No effect of the defined groups on weight gain was found.
Among the few studies conducted with non-pregnant individuals(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar7) and pregnant women(Reference Gontijo, Cabral and Balieiro15), the timing of food intake has been associated with eating patterns and diet quality, respectively. A recent study from our group with first trimester pregnant women found that the timing of the first eating episode had a negative association on diet quality and fruit consumption, but no association was found between the timing of the last eating episode and diet quality(Reference Gontijo, Cabral and Balieiro15). The present study found similar results and now evidences these results in a prospective way, throughout pregnancy. Also, we have shown the effect of the defined groups separated by the timing of the first and last eating episodes on eating patterns.
The results from our cohort highlight the link between first eating time and eating patterns and diet quality. Studies with non-pregnant individuals showed that those who consume the first eating episode earlier, supposedly breakfast, may feel less rushed and therefore probably consume a more adequate meal in terms of quality and quantity(Reference Murakami, Livingstone and Fujiwara35). This morning dietary pattern leads to better satiety and hunger control throughout the day(Reference Jakubowicz, Froy and Wainstein36,Reference Berti, Riso and Brusamolino37) , resulting in less food consumption at night. The adequate distribution of energy content throughout the day, with higher energy intake in the morning and midday and lower energy intake in the evening, seems to be linked with better diet quality(Reference Wittig, Hummel and Wenzler38), as found in the present study. Moreover, the greatest number of eating episodes during the day may be related to appropriate snacks between main meals, favouring the best diet quality(Reference Aljuraiban, Chan and Oude Griep16,Reference Poscia, Teleman and Azzolini39) , and more specifically, a higher number of eating episodes may be related to higher fruit intake by pregnant women(Reference Gontijo, Cabral and Balieiro15).
The adequate distribution of energy and macronutrients throughout the day, with a larger proportion of total daily energy consumed in the morning and less in the evening(Reference Jakubowicz, Barnea and Wainstein8), and the early time of main meals(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar7) also seem to be better for weight regulation in non-pregnant women. In contrast to these findings, the present study found no effect between the timing of the first and last eating episode and BMI and weight gain per week during pregnancy. Therefore, the effect of meal timing on weight regulation may differ between pregnant and non-pregnant women, especially considering that the studies which evaluated meal timing influences on weight loss in non-pregnant women focused on overweight and obese individuals in weight loss treatment(Reference Garaulet, Gómez-Abellán and Alburquerque-Béjar7,Reference Jakubowicz, Barnea and Wainstein8) . However, in the case of pregnant women, antenatal monitoring should provide adequate information for healthy weight gain in this period(Reference Rasmussen and Yaktine40). In addition, the weight gain during pregnancy is a result of complex developments in both the mother and fetus and involves components such as fetal weight gain, placental weight change, maternal body fat increases and alterations in extracellular volume(Reference Rasmussen and Yaktine40). However, further studies need to be performed to elucidate the relationship between meal timing and weight gain during pregnancy.
A systematic review of weight gain during pregnancy suggested that higher gestational weight gain is associated with increased energy intake during pregnancy but did not show a conclusive association with macronutrient intake(Reference Tielemans, Garcia and Peralta Santos41). However, none of the defined groups separated by the timing of the first and last eating episodes evaluated in our study changed their energy and macronutrient consumption during this period. Similar results have been reported in other studies(Reference Moran, Sui and Cramp42,Reference Talai Rad, Ritterath and Siegmund43) , which reinforce the tendency of many pregnant women to fail to reach the recommendation of increased energy intake during the second and third trimesters(44). Another possibility is that food intake has been underreported, especially in late pregnancy(Reference Moran, McNaughton and Sui45), but it is worth mentioning that food consumption results expressed as a percentage of energy intake may have not been affected by the exclusion of under-reporters(Reference Hirvonen, Männistö and Roos46), thus favouring the evaluation of food intake by the distribution of percentage energy intake throughout the day.
The higher total energy intake by the Early/Late group, compared with the Early/Early and Late/Early groups, found in the present study could be explained by the time of the last eating episode, since later feeding can provide a longer opportunity to eat(Reference Reid, Baron and Zee47). A greater eating duration, the interval between the first and last eating episodes of the day(Reference Gill and Panda29), may result in higher total daily energy intake(Reference Gill and Panda29,Reference Reid, Baron and Zee47) , which has been considered a negative factor for nutritional and metabolic health(Reference Gupta, Kumar and Panda48). However, our results suggest that it is important in nutritional interventions to consider not only the total energy content consumed but also the timing of food intake and the distribution of percentage of energy content consumed throughout the day.
Despite the differences between the defined groups separated by the timing of the first and last eating episodes regarding total energy and macronutrient consumption, no differences were found in the total score and in almost all BHEI-R components, which may be due to the fact that BHEI-R assesses the number of daily servings adjusted by the energy density (4184 kJ/day). Another consideration is that the BHEI-R evaluates the total consumption of the day, rather than individual meals, making it difficult to compare the data of this index with the difference found in the distribution of energy intake in the meals throughout the day.
The main finding of our cohort study needs to be considered in antenatal nutritional counselling, since the early timing of the first eating episode may be related to more adequate eating patterns with higher food intake in the morning and lower in the evening and better diet quality in terms of fruit components. A good diet quality(Reference Gadgil, Ehrlich and Zhu49) and meal timing(Reference Chandler-Laney, Schneider and Gower11–Reference Loy, Wee and Colega14) may also be considered a protective factor for maternal outcomes, such as glycaemic control. In this sense, pregnant women consuming less energy at night(Reference Loy, Cheng and Colega12) and more specifically, the reduction of carbohydrate intake at night(Reference Chandler-Laney, Schneider and Gower11), may be beneficial for glucose and insulin metabolism during pregnancy(Reference Chandler-Laney, Schneider and Gower11,Reference Loy, Cheng and Colega12) . In addition, pregnancy is a good opportunity to improve eating patterns and diet quality for better pregnancy results(Reference Shin, Lee and Song2–Reference Shapiro, Kaar and Crume4) and to encourage healthy lifestyle practices.
There are some limitations in our study that need to be considered. The evaluations were performed using questionnaires which, although previously validated in other studies, are subjective and dependent on the memory and motivation of the participants. To obtain accurate data, however, respondents were trained before participating in the survey and our team has been highly trained(Reference Althubaiti50). Although in the present study, the timing of the first and last eating episodes did not change during pregnancy, another limitation could be that we have not registered day/night duration (sunrise and sunset) concomitant with the application of the 24HR. The day/night duration (sunrise and sunset) can influence the timing of food intake because individuals should perform some activities, including food intake, during the light portion of the day(Reference Wright, McHill and Birks51). Further studies with pregnant women are needed to assess sunlight influence on the timing of food intake. In addition, circadian eating could also be influenced by sleep, but this association was not evaluated. Another limitation is that the practice of physical activity, which was used as an adjustment variable, was not assessed by a validated questionnaire. This method may not be accurately assessing the physical activity intensity and thus limiting the adjustment of the models by this variable. Lastly, our results are based on only 100 pregnant women, who had regular consultations in the public health care system, and the generalisation of results for all pregnant women cannot be made, especially with regard to high-risk pregnant women.
Conclusion
We conclude that the early eaters of the first eating episode have more adequate eating patterns, with a higher percentage of energy and carbohydrates consumed during morning meals and a lower level at evening meals. Early eaters of the first eating episode also have a better diet quality in terms of the total fruit and whole fruit components during pregnancy, when compared with the late eaters of the first eating episode. Our results suggest that the timing of food intake is a new variable to be considered in nutritional guidelines in antenatal care for promoting maternal–fetal health.
Acknowledgements
The authors thank all of the pregnant women who agreed to participate in this project.
The present study received financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasil (CNPq; grant number: 449938/2014-0) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; CBB – APQ-01154-14). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors’ responsibilities were as follows: C. A. G., L. C. T. B., W. M. F., C. A. C. and Y. C. P. M. conceptualised and designed the study; C. A. G., L. C. T. B., G. P. T. and W. M. F. collected the data; C. A. G., C. A. C. and Y. C. P. M. analysed and interpreted the data; C. A. G. wrote the initial manuscript; C. A. G., L. C. T. B., G. P. T, W. M. F., C. A. C. and Y. C. P. M. reviewed the manuscript and approved the final manuscript.
The authors declare no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114519003398








