The increase in global biofuel production and human utilisation, although controversial, has resulted in high prices of agricultural products, including maize( Reference Popp, Lakner and Harangi-Rákos 1 , Reference Zilberman, Hochman and Rajagopal 2 ), an important feed grain for livestock, which has increased the demand for alternative energy sources in animal feed.
Crude glycerin (CG), a co-product of biodiesel production, is an ingredient that can replace maize in ruminant diets, and its production has the potential for continuous growth in the future( Reference Quispe, Coronado and Carvalho 3 , Reference Benedeti, Silva and Paula 4 ). This by-product could increase the available energy for use by the animal, as it would be absorbed through the ruminal epithelium and used as a precursor of gluconeogenesis in the liver( Reference Remond, Souday and Jouany 5 ). The main effect of CG on ruminal fermentation is characterised by a reduction of the acetate:propionate ratio( Reference Avila, Chaves and Hernandez-Calva 6 , Reference Benedeti, Silva and Paula 4 ) and, when used up to 100 g/kg in Nellore steer diets, CG does not influence DM intake (DMI)( Reference Fávaro, Bertocco and D’Aurel 7 ).
Another alternative source used to increase the energy density in diets for increased animal production is lipids( Reference Zinn and Jorquera 8 , Reference Gomez, Granja-Salcedo and Castagnino 9 ). Lipid supplementation in Nellore has consistently been associated with an increase in microbial protein synthesis( Reference Messana, Berchielli and Arcuri 10 , Reference Fiorentini, Carvalho and Messana 11 ). Currently, improved N use efficiency in ruminants has received increased attention owing to the high cost of protein sources in animal feed and the environmental impact of excessive excretion of N from livestock production( Reference Lee, Hirstov and Heyler 12 ).
However, lipid supplementation could cause negative effects on the DMI and diet digestibility when the diethyl ether extract (EE) content exceeds 70 g/kg( Reference Fiorentini, Carvalho and Messana 11 ). Adverse effects of lipids on ruminal fermentation are mainly owing to the toxicity of unsaturated fatty acids (UFA) to ruminal microbiota, especially cellulolytic bacteria( Reference Maia, Chaudhary and Figueres 13 ). However, CG could partially inhibit ruminal lipolysis and modulate the release of UFA in the rumen( Reference Krueger, Anderson and Tedeschi 14 – Reference Granja-Salcedo, Souza and Dias 16 ), and could thereby reduce the deleterious effects of UFA on ruminal fermentation. Thus, CG associated with lipids could be an alternative energy source and could constitute a useful strategy for the partial replacement of maize in cattle diets.
Previous studies have reported beneficial effects of CG associated with soyabean oil (SO) in Nellore bull diets, such as greater daily gain and feed efficiency( Reference Silva, Lage and San Vito 17 ), without adversely affecting the DMI and ruminal fermentation parameters( Reference Gomez, Granja-Salcedo and Castagnino 9 ) and increased UFA duodenal flow( Reference Granja-Salcedo, Souza and Dias 16 ). However, few studies have examined the effects of CG and lipid combination on ruminal fermentation, the ruminal microbial population and N utilisation in high-lipid-content diets (>70 g EE/kg MS).
Thus, the objective of this study was to determine whether a combination of CG and SO could be used to partially replace maize in the diet of Nellore steers while maintaining optimum feed utilisation. Our hypothesis was that diets with high lipid content and the addition of CG could partially replace maize without compromising adversely the ruminal microbiota, DMI, digestibility and ruminal metabolism.
Methods
Animals, experimental design and diets
This study was carried out in strict accordance with the recommendations in the Brazilian College of Animal Experimentation (Colégio Brasileiro de Experimentação Animal) guidelines. The protocol was approved by the Ethics, Bioethics and Animal Welfare Committee (Comissão de Ética e Bem Estar Animal) of Facultade de Ciencias Agrarias e Veterinarias (FCAV) of the Universidade Estadual Paulista (UNESP), Jaboticabal campus, Brazil (protocol no. 07784/14). Ruminal and duodenal cannulation was conducted 8 months before the experiment under xylazine sedation and local anaesthesia with lidocaine hydrochloride, and all efforts were made to minimise suffering.
The experiment for the evaluation of ruminal fermentation and microbial population was conducted at the Experimental Feedlot of the Animal Science Department in the UNESP, Jaboticabal, Brazil.
Eight Nellore steers (mean body weight 501·3 (sem 18) kg), castrated and fitted with ruminal silicone-type ruminal cannulas and duodenal T-type cannulas (Kehl®), were used in a double, simultaneous, Latin square design 4×4 with a 2×2 factorial arrangement of treatments (with or without SO and with or without CG). The experiment consisted of four consecutive periods of 21 d each. Each period consisted of 14 d adaptation, 5 d faeces and urine collection and 1 d sampling of ruminal fluid.
The animals were fed with experimental diets (Table 1) and a mineral supplement at 100 g/animal (containing per kg: Ca, 146 g; P, 40 g; S, 40 g; Na, 130 g; Cu, 1·35 g; Mn, 1·04 g; Zn, 5 g; iodine, 100 mg; Co, 80 mg; Se, 26 mg; F, ≤800 mg) twice per d at 06.00 and 16.00 hours as total mixed ration. Throughout the entire experimental period, the allowance was adjusted to allow refusals of approximately 5 % of the total amount consumed on the previous day.
Table 1 Proportion of ingredients and bromatological composition of experimental diets

SO, soyabean oil; CG, crude glycerine; RDP, rumen degradable protein; NFC, non-fibrous carbohydrates; ME, metabolisable energy.
* 60 g/kg of SO in DM diet.
† 100 g/kg of CG in DM diet.
Experimental diets contained Tifton 85 hay, ground maize, soyabean meal, urea and 60 g/kg DM of SO combined with 100 g/kg DM of CG; or 60 g/kg DM of SO without CG; or 100 g/kg DM CG without SO; or without CG and SO. Experimental diets were formulated according to recommendations of the Agricultural and Food Research Council( 18 ), had a roughage:concentrate ratio of 30:70, were isonitrogenous and were prepared to be consumed within 4 d to prevent the oxidation of dietary ingredients. The CG (glycerol: 803·4 g/kg; EE: 15·9 g/kg; ash: 50·3 g/kg; water: 120·2 g/kg) was obtained from biodiesel production based on SO (Cargill). The SO used contained 121·6 g/kg stearic acid; 301·6 g/kg oleic acid; 347·83 g/kg linoleic acid; and 2·29 g/kg linolenic acid.
Feed intake and apparent digestibility
On days 15–21 of each experimental period, before the morning feeding when subsamples of approximately 100 g/kg were obtained and frozen at −20°C, the feed refusals were collected and weighed daily. At the end of each experimental period, composite samples were collected for each animal based on the feed refusal weight.
Faeces were collected on days 16–20 of each experimental period to estimate the apparent digestibility of dietary constituents. Samples were collected daily immediately after each spontaneous defecation, and stored in 20-litre buckets. After each 24-h collection period, the total amount of faeces produced per animal was weighed and homogenised; from this, a sample (15 % of daily faecal production; approximately 200 g) was removed and dried. For each animal, in each experimental period, we formed a faecal-composite sample based on the pre-dried weight.
To measure organic matter (OM) apparently digested in the rumen (OMDR), duodenal samples (150 ml) were obtained by duodenal cannula collected at 6-h intervals on days 19 and 20 of each experimental period. The sample collection during the 2nd day was delayed for 3 h to ensure that every 3-h slot in a 24-h period was properly represented. Indigestible neutral-detergent fibre (NDF), an indicator of the daily flow of DM in the duodenum( Reference Harvatine and Allen 19 ), was assessed via in situ incubation of feeds offered, feed refusals, faeces and duodenum samples for 288 h( Reference Valente, Detmann and Valadares Filho 20 ). After the incubation time, bags were removed and washed in running water until the water was clear. Next, samples were pre-dried at 55°C for 72 h and used for NDF determination, and duodenal DM flow was calculated according to the following equation: (faecal DM×g/kg indigestible NDF in faecal DM)/g/kg indigestible NDF in duodenal DM.
Feeds, feed refusals, faeces and duodenal samples were dried in a forced-air-circulation oven at 55°C for 72 h and ground in a Wiley mill (Thomas Scientific) to pass a 1-mm screen. Samples were analysed for DM (Method 934.01), ash (Method 942.05) and EE (Method 920.39) according to Association of Official Analytical Chemists( 21 ). N was determined using a LECO FP-528 N analyzer (LECO Corp.) and the N values were converted to crude protein (CP) by multiplying by 6·25. Gross energy was obtained by the combustion of samples in an adiabatic bomb calorimeter (IKA model 2000 Basic; IKA Corp.). NDF determination was conducted using α-amylase without the addition of sodium sulphite, following the recommendations of Van Soest et al. ( Reference Van Soest, Robertson and Lewis 22 ), and adapted for the ANKOM 200 Fiber Analyzer (ANKOM Technology Corporation). Acid-detergent fibre was determined using the method described by Goering & Van Soest( Reference Goering and Van Soest 23 ) and adapted for the ANKOM 200 Fiber Analyzer. Acid-detergent lignin was determined by solubilisation of cellulose with sulphuric acid according to Van Soest & Robertson( Reference Van Soest and Robertson 24 ). Starch was determined enzymatically according to Bach Knudsen et al. ( Reference Bach Knudsen, Eggum and Jacobsen 25 ). Total carbohydrates and non-fibrous carbohydrate (NFC) values were calculated as described by Sniffen et al. ( Reference Sniffen, O’Connor and Van Soest 26 ). Ruminal degradable protein was calculated as described by Ørskov & McDonald( Reference Ørskov and McDonald 27 ).
Ruminal fermentation parameters
Ruminal fluid was sampled at day 21 of each experimental period at 0, 3, 6, 9, 12, 15 and 18 h after the morning feeding. An amount of 50 ml of ruminal fluid was taken after filtration through cotton double fabric, and then pH was measured. Two aliquots of 25 ml were stored at −20°C and later used to determine ammonia N (NH3-N) and volatile fatty acid (VFA) concentration. NH3-N was determined in duplicate following the methodology of Fenner( Reference Fenner 28 ) adapted for use in Kjeldahl distillation. The VFA concentration was quantified by GC (GC Shimatzu model 20−10, with automatic injection; Shimatzu Corporation) using a SP-2560 capillary column (30 m×0·25 mm diameter, 0·02 mm thick; Supelco) according to the methodology described by Palmquist & Conrad( Reference Palmquist and Conrad 29 ).
Ruminal micro-organisms
On day 21 of the trial period, samples of ruminal content were collected before the morning feeding. Samples of approximately 60 g/animal (a mix of liquid and solid) from the dorsal, central and ventral regions of the rumen were collected through the ruminal cannula, immediately frozen and stored at −80°C for DNA extraction.
A Fast DNA SPIN Kit for Soil (MP Bio®; Biomedicals) extraction kit was used to extract metagenomic DNA from 200 mg of sample according to the manufacturer’s instructions. DNA concentrations were measured fluorometrically (Qubit® 3.0, kit Qubit® dsDNA Broad Range Assay Kit; Life Technologies) and DNA purity was assessed spectrophotometrically (NanoDrop® ND-1000 Spectrophotometer; Thermo Fisher Scientific) at A260:A230 nm and A260:A280 nm ratios. Integrity was determined by agarose gel electrophoresis using a 0·5 % (w/v) gel, and subsequent staining with ethidium bromide (5 mg/ml).
Bacterial diversity analysis was performed on selected samples from animals of the first Latin square 4×4 (a total of sixteen samples; four of each experimental group). Primers for PCR (515F=5'-GTGNCAGCMGCCGCGGTAA-3' and 926R=5'-CCGYCAATTYMTTTRAGTTT-3') and sequencing used in this analysis were described by Caporaso et al. ( Reference Caporaso, Lauber and Walters 30 ). Each sample was amplified in triplicate, and each PCR reaction mixture (20 μl final volume) contained 20 ng of DNA, 10 μm of each forward and reverse primer, 1·25 mm magnesium chloride, 200 μm dNTP mix (Invitrogen), 1·0 U platinum Taq DNA polymerase high fidelity (Invitrogen), a high-fidelity PCR buffer (1×) (Invitrogen) and Milli-Q water (Milli-Q® Type 1 Ultrapure Water Systems). Reactions were conducted at 95°C for 3 min to denature the DNA, with amplification proceeding for forty cycles at 95°C for 30 s, 53·8°C for 30 s and 72°C for 45 s; a final extension of 10 min at 72°C was added to ensure complete amplification.
The purity of PCR products was verified by agarose gel (1 %) electrophoresis and the amplicon size was estimated by comparison with a 1 Kb plus DNA ladder (Invitrogen). The PCR fragments were purified using the Zymoclean™ Gel DNA Recovery kit (Zymo Research) according to the manufacturer’s instructions. Composite samples for sequencing were created by combining equimolar ratios of amplicons from the triplicate samples. Sequencing was performed using the Ion Torrent Personal Genome Machine (PGM™; Thermo Fisher Scientific) with the Ion 314™ Chip Kit v2 (Thermo Fisher Scientific) at the Sequencing Facility, Department of Technology of FCAV, Jaboticabal, Brazil.
Sequence trimming was carried out by Prinseq version 0.20.3 selecting sequences over 200 bp in length with an average quality score greater than 25 based on Phred quality. Sequences were clustered using Mothur 1.36.1( Reference Schloss, Westcott and Ryabin 31 ) and taxonomic assignment of sequences was performed against the SILVA database( Reference Pruesse, Quast and Knittel 32 ). Sequence data were grouped into operational taxonomic units that shared>97 % sequence similarity and were summarised at phylum and genera levels.
Quantitative PCR (qPCR) was used to identify and quantify Archaea abundance, with a total bacterial primer set recommended by Denman & McSweeney( Reference Denman and McSweeney 33 ) (forward 5'-CGGCAACGACAACCC-3' and reverse 5'-CCATTGTAGCACCTGTGTAGCC-3'); a different set of primers was used for Archaea (forward 5'-TTCGGTGGATCDCARAGRGC-3' and reverse 5'-GBARGTCGWAWCCGTAGAATCC-3') as used by Denman et al. ( Reference Denman, Tomkins and McSweeney 34 ).
All forward and reverse primers used were tested in four concentrations (200, 400, 600 and 800 nm) to determine the minimum concentration of primer with the lowest threshold cycle (C t ), and reduce non-specific amplifications. We determined the slope value and calculated the efficiency. The validation of the selected primer concentrations was performed with different concentrations of DNA (150, 125, 100, 50, 25 and 12·5 ng).
The qPCR (final volume reactions 12·5 µl) contained 6·25 µl of SYBR Green PCR Master Mix (Bio-Rad), 200 (Archaea) or 600 nm (total bacteria) of each primer pair, ultrapure water (Milli-Q; Millipore Corporation) and 100 ng of the DNA. Reactions were performed in triplicate in an Applied Biosystems 7500 Real-time PCR System (Thermo Fisher Scientific). Negative controls contained all reaction components except DNA. ROX (ROX™/Texas Red®; Thermo Fisher Scientific) was used as a passive reference dye.
PCR conditions were as follows: amplification at 50°C for 2 min, 95°C for 10 min; 35 denaturing cycles at 95°C for 15 s; annealing at 60°C for 60 s; and extension at 78°C for 1 min. After the amplification cycle, a step was added, increasing the temperature from 60 to 95°C, to obtain a dissociation curve of the reaction products, and analyse the specificity of the amplification.
Changes in targeted populations were calculated using a relative quantification calculation and the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method, with the control period used as the calibrator and total bacterial(
Reference Denman and McSweeney
33
)
C
t
(cycle threshold) values used as the reference value(
Reference Livak and Schmittgen
35
). Thus, results were expressed as a proportion of the 16S rRNA associated with total bacteria.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method, with the control period used as the calibrator and total bacterial(
Reference Denman and McSweeney
33
)
C
t
(cycle threshold) values used as the reference value(
Reference Livak and Schmittgen
35
). Thus, results were expressed as a proportion of the 16S rRNA associated with total bacteria.
For the quantification of ruminal ciliate protozoa, samples of ruminal content were collected via the cannula on day 21 of the trial period 3 h after the morning feeding, and ruminal content aliquots were preserved in formalin (a 1:1 solution of water and 370 ml/l formaldehyde) as described by D’Agosto & Carneiro( Reference D’Agosto and Carneiro 36 ). Direct identification and counts of protozoa were performed in a Sedgewick Rafter counting chamber( Reference Dehority 37 ). The samples were diluted with 200 ml of glycerol/l and stained using Lugol’s solution before counting the cells( Reference D’Agosto and Carneiro 36 ).
Statistical analyses
Statistical analyses were performed using R Software version 3.2.2 (2015; R Core Team). Initially, the mathematical assumptions of data were tested (Shapiro–Wilk test and Bartlett test).
Data of intake, digestibility, OMRD and ruminal protozoa were compared between treatments by ANOVA as a double 4×4 Latin square design (four treatments and four periods), balanced for residual effects, in a factorial arrangement (A×B). The fixed effect of factor A corresponded to the SO addition (yes/no) and factor B to the CG addition (yes/no), factors interactions, treatments error, the random effects of Latin square, period, animal and residues corresponding to the model.
Data regarding pH, NH3-N and VFA were compared between treatments and time by repeated-measures ANOVA in a double 4×4 Latin square design (four treatments and four periods), balanced for residual effects, in a split-plot factorial arrangement (A×B). The model included the fixed effects of factor A (corresponded to the SO addition, yes/no) and factor B (corresponded to the CG addition, yes/no) that were considered as independent variables; the sampling times considered as dependent variable (covariate), factors interactions, treatments residues, factors and time interactions; and the same random effects of the previous model.
Tukey’s post hoc test was applied when ANOVA indicated a significant difference, considering statistical significance when P≤0·05.
Bacterial and Archaea abundance was compared between experimental groups (oil and glycerin; oil and no glycerin; no oil and glycerin; and no oil and no glycerin) using Kruskal–Wallis and Dunn’s post hoc tests.
Results
Feed intake and digestibility
All diets allowed a similar intake of DM, OM, CP and NDF when expressed as % of animal body weight (P>0·05; Table 2). However, when the intake was expressed as kg/d, there was an interaction (P<0·05) between SO and CG on DMI, CP, NFC and GE intake; thus, steers supplemented with oil without glycerin showed the lowest intake of DM and CP (P<0·05). Therefore, steers fed with oil and glycerin in the diet presented similar DMI and CP intake than steers fed a no oil and no glycerin diet (P=0·216). Diets with an oil addition reduced the NDF intake (P=0·049) but increased the EE intake (P<0·001). The highest NFC intake was presented by animals fed a no-oil and no-glycerin diet (P<0·05), and the no-oil and glycerin addition diet generated the lowest GE intake (P<0·001).
Table 2 Nutrient intake, total apparent digestibility and nitrogen faecal outputs in feedlot Nellore steers fed diets containing or not containing soyabean oil (SO) with or without crude glycerine (CG)
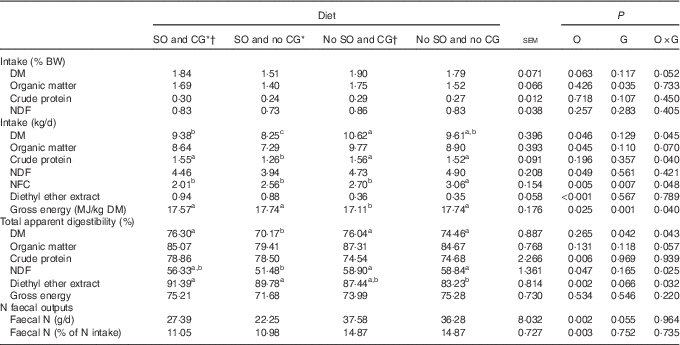
O, SO addition effect; G, CG addition effect; BW, body weight; NDF, neutral-detergent fibre; NFC, non-fibrous carbohydrates.
a,b,c Values on the same row with unlike superscript letters were significantly different (P<0·05), as obtained with Tukey’s test.
* Addition of 60 g/kg of SO in DM diet.
† Addition of 60 g/kg of SO for 100 g/kg of CG in DM diet.
There was an interaction effect of SO and CG on DM, NDF and EE total apparent digestibility. Thus, diet containing oil and no glycerin presented the lowest DM and NDF digestibility (P<0·05). EE digestibility was lowest for the diet with no oil and no glycerin when compared with diets containing SO (P=0·001). Steers fed diets containing SO showed greater CP digestibility (P<0·001).
Steers fed diets containing SO had less total faecal N excretion expressed as g/d (P=0·002; Table 3) and % of N intake (P=0·003).
Table 3 Rumen fermentation parameters in feedlot Nellore steers fed diets containing or not containing soyabean oil (SO) with or without crude glycerine (CG)
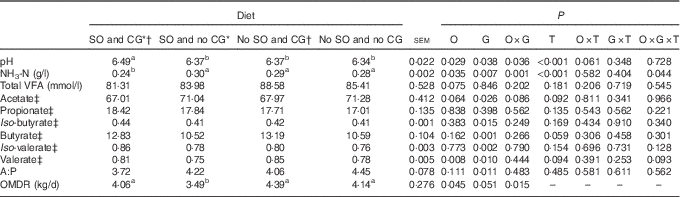
O, SO addition effect; G, CG addition effect; T, time effect; VFA, volatile fatty acid; A: P, acetate:propionate ratio; OMDR, organic matter degraded into rumen.
a,b Values on the same row with unlike superscript letters were significantly different (P<0·05), as obtained with Tukey’s test.
* Addition of 60 g/kg of SO in DM diet.
† Addition of 60 g/kg of SO for 100 g/kg of CG in DM diet.
‡ Expressed as mmol/100 mol of total VFA.
Ruminal fermentation parameters
The addition of SO, CG and sampling time affected ruminal NH3-N concentration (P<0·05; Fig. 1); steers fed SO and glycerin addition diet had the lowest ruminal NH3-N concentration at 3, 6 and 12 h after feeding. In addition, SO and CG affected the average ruminal pH and NH3-N concentration (Table 3); steers fed an oil and glycerin diet had the highest ruminal pH and lowest ruminal NH3-N concentration (P<0·05). The total VFA concentration and propionic (P) proportion was not influenced by the diets (P>0·05). However, diets containing CG led to a less proportion of acetate (A) and A:P ratio and greater proportions of iso-butyrate, butyrate, iso-valerate and valerate (P<0·05).
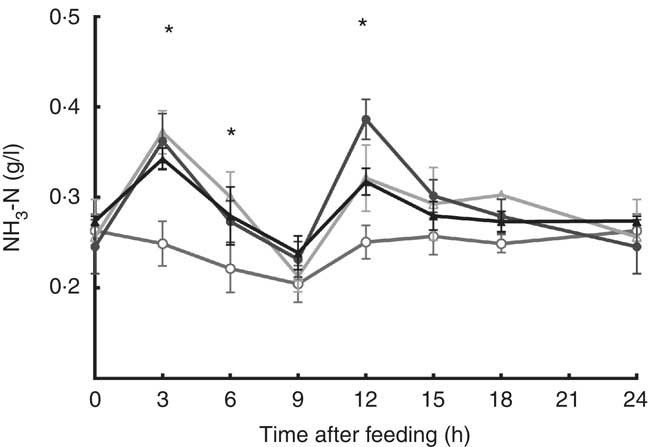
Fig. 1 Ruminal ammonia N (NH3-N) concentration during a 24-h period in Nellore steers fed diets containing or not containing 60 g/kg of soyabean oil in DM diet (SO) with or without 100 g/kg of crude glycerine in DM diet (CG). Values are means with their standard errors. ![]() , SO and CG;
, SO and CG; ![]() , SO and no CG;
, SO and no CG; ![]() , no SO and CG;
, no SO and CG; ![]() , no SO and no CG. * SO and CG diet results in a lowest ruminal NH3-N concentration (P<0·05) as obtained with Tukey’s test.
, no SO and no CG. * SO and CG diet results in a lowest ruminal NH3-N concentration (P<0·05) as obtained with Tukey’s test.
Ruminal micro-organisms
The number of the generated sequences was not affected by the diet (P=0·639). In all, fifteen phyla were identified and 13 % of the sequences could not be classified at the phylum level. Firmicutes and Bacteroidetes were the most abundant phyla and accounted for >76 % of the total bacterial community in all samples sequenced.
The abundance of the genera Fibrobacter, Butyrivibrio, Ruminococcus, Lachnospiracea incertae sedis and Pseudobutyrivibrio was less in the rumen of steers fed diet with oil without glycerine (Table 4; P<0·05). In contrast, Acinetobacter was most abundant in steers fed diets containing SO (P=0·001).
Table 4 Ruminal bacteria composition and methanogen (Archaea) abundance in feedlot Nellore steers fed diets containing or not containing soyabean oil (SO) with or without crude glycerine (CG) (Medians and interquartiles)
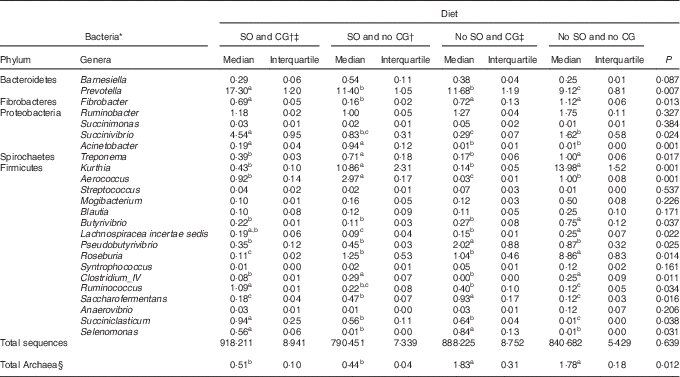
a,b,c Median values on the same row with unlike superscript letters were significantly different (P<0·05), as obtained using Dunn’s test.
* Expressed as a proportion of operational taxonomic units identified.
† Addition of 60 g/kg of SO in DM diet.
‡ Addition of 60 g/kg of SO for 100 g/kg of CG in DM diet.
§ Measured based on the proportion of the specific 16S rRNA associated with total bacteria.
Independently of the addition of oil, CG negatively affected Treponema, Kurthia, Roseburia and Clostridium_IV populations (P<0·05), but stimulated Selenomonas abundance (P=0·001). Animals fed no oil and CG in the diet showed greater ruminal populations of Pseudobutyrivibrio (P=0·025).
Steers fed oil and glycerin as partial replacement of maize in the diet had a greater ruminal abundance of Prevotella, Succinivibrio, Ruminococcus and Succiniclasticum (P<0·05).
Ruminal Archaea abundance was affected by diets containing SO (P=0·012). Thus, the diets of oil and glycerin and oil and no glycerin addition decreased the Archaea proportion by four and three times, respectively.
Total ruminal ciliate protozoa were less in steers fed diets containing SO (Table 5; P=0·011). The addition of oil decreased the abundance of Entodinium, Dasytricha, Isotricha, Diploplastron, Eudiplodinium and Polyplastron genera. In contrast, when CG was used, the abundance of Diploplastron increased (P=0·034).
Table 5 Ruminal ciliate protozoa concentration in feedlot Nellore steers fed diets containing or not containing soyabean oil (SO) with or without crude glycerine (CG)
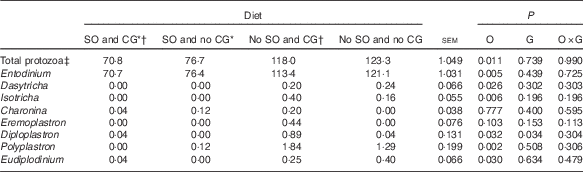
O, SO addition effect; G, CG addition effect.
* Addition of 60 g/kg of SO in DM diet.
† Addition of 60 g/kg SO for 100 g/kg of CG in DM diet.
‡ Expressed as no. of protozoa×104/ml of rumen liquid.
Discussion
In this study, we evaluated the ability of diets with high lipid content and CG to partially replace dry ground maize for Nellore steer feed by examining the effects of this diet on nutrient intake and digestibility, ruminal fermentation parameters and ruminal microbiome. Steers fed SO and CG to partial replacement of ground showed similar DMI, DM, OM and NDF digestibility to that of steers fed a diet without oil or glycerin. Thus, the hypothesis that diets with high lipid content (>70 g EE/kg DM) and supplemented with CG could partially replace maize diets without compromising intake, digestibility and ruminal fermentation was accepted.
In ruminants, negative effects on DMI and ruminal fermentation are often reported when they are fed diets including vegetable oils( Reference Yang, Bulb and Wang 38 , Reference Wanapat, Mapato and Pilajun 39 , Reference Fiorentini, Carvalho and Messana 11 ). As expected, the diet with oil and no glycerin addition (89 g EE/kg DM) decreased the DMI and NDF intake and digestibility. These observations may be linked to the high content of EE of this diet, especially the high proportion of UFA with the SO that negatively affected the ruminal microbes, including fibre-degrading (e.g. Fibrobacter, Butyrivibrio, Lachnospiracea incertae sedis and Ruminococcus) and starch-degrading (e.g. Ruminobacter, Pseudobutyrivibrio, Roseburia and Syntrophococcus) bacteria, as well as total ruminal ciliate protozoa. UFA could directly disrupt microbial cell membranes and cellular function( Reference Maia, Chaudhary and Figueres 13 ), coat bacteria and feed particles in the rumen in lipids( Reference Jenkins 40 ), and directly inhibit ruminal protozoa( Reference Oldick and Firkins 41 , Reference Yang, Bulb and Wang 38 ) and Archaea( Reference Dohme, Machmuller and Wasserfallen 42 ). Consequently, animals fed the SO and no glycerin addition diet showed a marked reduction in OMDR, which represented the lowest DMI and NDF intake and digestibility.
However, animals fed oil and glycerin addition (86 g EE/kg DM) showed similar DMI, DM and NDF digestibility to that of animals fed without SO or CG supplementation (no oil and no glycerin diet: 33 g EE/kg DM). These findings suggest the potential of glycerin as a partial inhibitor of ruminal lipolysis( Reference Krueger, Anderson and Tedeschi 14 – Reference Granja-Salcedo, Souza and Dias 16 ); consequently, the modulation of UFA liberation in the rumen could reduce the adverse effects of UFA on ruminal micro-organisms and ruminal fermentation. It is possible that glycerin has a protective effect in diets with high lipid content and ameliorated the effects of lipids on ruminal fermentation( Reference Jenkins and Bridges 43 , Reference Fiorentini, Carvalho and Messana 11 ).
The similar OMDR between steers fed oil and glycerin to partial replacement of ground and steers fed a diet without oil or glycerin, and the ruminal microbial profile of animals fed oil and glycerin diet, supports these results; SO and CG addition did not affect the abundance of important cellulolytic bacteria (Lachnospiracea incertae sedis and Ruminoccocus) and increased the abundance of some amylolytic/proteolytic bacteria (Prevotella, Succinivibrio, Succiniclasticum and Selenomonas).
Supplementation with oil and glycerin could replace 35 % of the ground maize used in the unsupplemented diet. Clearly, the lowest dietary starch (190 g starch/kg DM) resulted in a greater mean ruminal pH. However, although all diets contained a high proportion of concentrate, the average pH was adequate (pH>6·0) for the maintenance of cellulolytic bacteria and ciliated protozoa in the rumen( Reference Russell and Dombrowski 44 , Reference Granja-Salcedo, Ribeiro Júnior and de Jesus 45 ).
Inclusion of CG in the current study altered the ruminal VFA proportions, decreased acetate and increased butyrate ruminal concentrations. These results are consistent with those of other studies on cattle fed CG( Reference Shin, Wang and Kim 46 , Reference Paiva, Del Valle and Jesus 47 ), suggesting that glycerin is associated with shifts in the microbial genera in the rumen. For example, it increases the abundance of butyrate-producing bacteria (e.g. Pseudobutyrivibrio)( Reference Kopečný, Zorec and Mrázek 48 ) and Selenomonas, which is a bacteria capable of fermenting glycerin that is considered a secondary fermenter, converting lactate and glucose to propionate or valerate( Reference Marounek, Fliegrova and Bartos 49 , Reference Stewart, Flint and Bryant 50 ).
Although the ruminal propionate proportion did not differ significantly between the diets, the A:P ratio was less in animals fed an oil and glycerin diet, as a reflection of changes on microbial profiles that reduced ruminal acetate. Interestingly, when animals were fed an oil and glycerin diet to partial replacement of ground, the abundance of three gram-negative bacteria (e.g. Prevotella, Succinivibrio, and Succiniclasticum), involved in ruminal propionate production, increased. The cooperative interaction between these three bacterial genera may have a synergistic effect on digestion and reduce acetate. The genus Prevotella can degrade polysaccharides such as xylan, hemicellulose, pentose and arabinose; has proteolytic and amylolytic abilities; and produces acetate, succinate and propionate as ruminal fermentation products( Reference Stewart, Flint and Bryant 50 ). In addition, Succinivibrio attacks dextrin, pectin and some sugars and forms succinate, lactate, acetate and formate( Reference Bryant and Small 51 ), and Succiniclasticum specialises in fermenting succinate and converting it to propionate( Reference Van Gylswyk 52 ).
Moreover, both diets with CG addition induced a greater ruminal concentration of valerate, iso-valerate and iso-butyrate– fatty acids resulting from ruminal degradation of branched amino acids that are essential growth factors for cellulolytic bacteria( Reference Russell, O’Connor and Fox 53 ), as these fatty acids are important precursors of microbial odd and branched-chain fatty acids that characterise ruminal bacterial composition( Reference Kaneda 54 ).
The greater ruminal ammonia concentration observed at the 1st hours are likely related to dietary urea used in all diets, once urea is quickly converted to NH3-N in the rumen, which can increase NH3-N levels faster than its use by rumen microbiota( Reference Benedeti, Silva and Paula 4 ). However, ruminal ammonia variations by lipid supplementation can be attributed to changes in protein degradation or synthesis of microbial protein( Reference Doreau and Ferlay 55 ). In our study, diets with SO supplementation showed similar CP digestibly, but oil and glycerin diet allowed a greater CP intake than the diet with oil and without glycerin, and lowest ruminal NH3-N concentration was observed in animals fed oil and glycerin diet at the 1st hours after morning and afternoon feeding. These findings suggest that oil and glycerin addition in the diet allowed a better N: energy synchrony at 1st hours after feeding; this could result in enhanced assimilation of N by the ruminal ammonia-utilising species; consequently, oil and glycerin combination could enable a greater microbial protein flow.
The great decrease in total ruminal protozoa with diets containing SO was expected, because UFA have been reported to directly inhibit ruminal protozoa( Reference Oldick and Firkins 41 ). In addition, oil supplementation improves CP digestibility, which may be associated with the low protozoa counts in these diets, as ruminal protozoa populations have a negative effect on N utilisation in ruminants( Reference Ivan, Mir and Koenig 56 ). Results of previous studies in bovines showed that oil supplementation can decrease the protozoa abundance and increase the proteolytic bacteria( Reference Loor, Ueda and Ferlay 57 , Reference Yang, Bulb and Wang 38 ).
On the other hand, hindgut fermentation in cattle has about 14 % of the capacity for fermentation of the rumen, and becomes more important with high grain feeding owing to increased flow of undigested substrate into the hindgut( Reference Gressley, Hall and Armetano 58 ). Therefore, N could also be recycled into the hindgut contributing to improve CP digestibility in steers fed diets containing SO. Once in ruminants fed high-concentrate diets, the depression of diet degradation in the rumen could be balanced by compensatory action of the hindgut( Reference Granja-Salcedo, Ribeiro Júnior and de Jesus 45 , Reference Metzler-Zebeli, Schmitz-Esser and Klevenhusen 59 ).
It is possible that greater CP digestibility was the determining factor that reduced the faecal N excretion in animals fed both diets containing SO. Nevertheless, from an environmental point of view, an important difference between the N lost with urine and that lost with faeces is that the main form of N in urine is urea( Reference Rom and Dahl 60 ); the ammonia that is emitted from livestock manure is mainly a breakdown product of urinary urea. The faecal N is more stable and contributes little to N emissions( Reference Satter, Klopfenstein and Erickson 61 ).
The ruminal Archaea abundance between both no-oil diets was similar, in accordance with other studies on cattle, which have reported no effects on Archaea abundance when CG was added to diets( Reference Danielsson, Werner-Omazic and Ramin 62 , Reference San Vito, Messana and Castagnino 63 ). However, when SO was added, the ruminal Archaea abundance decreased; this may be owing to different factors. Some of these factors could be as follows: Archaea are susceptible to UFA( Reference Dohme, Machmuller and Wasserfallen 42 ); as oil can also reduce the H concentration in the rumen by biohydrogenation, it could act as a H sink and thereby reduce Archaea activity( Reference Czerkawski 64 ); and the reduction in the total number of protozoa could have led to the decrease in the abundance of Archaea, as Archaea works symbiotically with protozoa to participate in H transfer.
A certain proportion of methanogens are associated with protozoa( Reference Jouany 65 ) and a reduction in protozoa abundance is in most cases indicative of a reduction in methane emission( Reference Guyader, Eugène and Nozière 66 ). Together, these results suggest that such dietary supplementation might lead to a reduction in methane emissions; future studies are required to confirm this inference.
Conclusion
CG associated with SO could be used as an energy source, and is a useful partial replacement for maize in cattle diets, resulting in a reduction of the faecal N excretion and ruminal methanogen abundance. This is environmentally beneficial, as methane emissions could be reduced without affecting dietary intake and digestibility, even when high lipid content is added to the diet.
Acknowledgements
The authors would like to thank Dr Eliana Gertrudes Macedo Lemos for providing the ultra-freezer for rumen sample storage and Dr Ricardo Ramirez-Uscategui for conducting statistical analysis.
This work was supported by the National Council of Technological and Scientific Development (CNPq – 475844/2012-2) and the São Paulo Research Foundation (FAPESP – 2013/23851-6 and 2015/17966-0).
The authors’ contributions are as follows: research questions – Y. T. G.-S. and T. T. B.; designing the study – Y. T. G.-S., J. D. M. and T. T. B.; carrying it out – Y. T. G.-S., V. C. d. S., A. V. L. D.; laboratorial analyses – Y. T. G.-S., A. V. L. D., L. R. R.; analysing the data – Y. T. G.-S. and L. T. K; writing the article – Y. T. G.-S., J. D. M., V. C. d. S. All authors provided feedback on the manuscript.
None of the authors has any conflicts of interest to declare.








