Glycomacropeptide (GMP) is a C-terminal fragment of κ-casein (residues 106–169) released by the endopeptidase chymosin (rennin). During commercial cheese making, this peptide is released into whey at a concentration of approximately 600 mg/l(Reference Nakano, Silva-Hernandez and Ikawa1, Reference Silva-Hernandez, Nakano and Ozimek2). Oral GMP stimulates cholecystokinin (CCK), a candidate satiety hormone, which may make this protein a useful component of a weight loss diet because CCK slows gastric emptying, which may in turn promote satiety(Reference Mangel and Koegel3–Reference Degen, Matzinger, Drewe and Beglinger5). GMP has also been detected in the blood of volunteers after milk or yogurt ingestion, suggesting that GMP can be formed in the gut and can be absorbed intact into intestinal cells(Reference Chabance, Marteau and Rambaud6). We have shown previously that GMP was associated with reduced fat mass in Wistar rats fed ad libitum for 7 weeks with diets differing in protein-type amount(Reference Royle, McIntosh and Clifton7). In addition, it has been found that GMP (33 kJ) had no effect on satiety or on food intake 75 min after consumption, but it did reduce daily food intake(Reference Burton-Freeman8). However, Gustafson et al. (Reference Gustafson, McMahon and Morrey9) reported no effect of GMP on subjective satiety or food intake at a test meal after the consumption of 0·4 and 2·0 g GMP in a 33 kJ preload; however, CCK was not measured, and various study design procedures, such as delivery with a low energy load, may have influenced the negative findings.
GMP naturally exists as a mixture of different glycoforms due to the carbohydrates sialic acid (N-acetylneuraminic acid, galactose, galactosamine and glucosamine attached to the peptide by O-glycosidic linkages. There have been no reports showing the effect of these natural GMP glycoforms on CCK, subjective measures of satiety and food intake. The objective of the present study was therefore to examine the effect of different GMP glycoforms on CCK, subjective measures of satiety and food intake.
Methods
GMP fractions from bovine cheese whey were manufactured using simulated moving bed chromatography on a Continuous Separations (Calgon Carbon Corporation, Pittsburgh, PA, USA) platform. This platform consisted of thirty columns of 4·6 cm diameter and 10 cm bed height packed with Pharmacia Q Sepharose Big Beads ion exchange resin (GE Healthcare, Uppsala, Sweden). The columns were equilibrated with reverse-osmosis water (pH 4·0) in the adsorption wash zone, followed by counter-current loading of the whey (pH 4·0; Bega Cheese Co., Bega, NSW, Australia). The columns were then rinsed with reverse-osmosis water at a neutral pH, and the glycosylated and minimally glycosylated GMP fractions were desorbed from the resin with 0·3 m-NaCl at pH 7·7 and was ultrafiltered using a spiral wound membrane (10 000 Da; Koch Membrane Systems, Inc., Wilmington, MA, USA). The flow through whey was ultrafiltered as described above to produce GMP-depleted whey protein concentrate. All samples were spray dried, and the N-acetylneuraminic acid, galactosamine and galactose contents of the glycosylated and minimally glycosylated GMP fractions were determined following a method that was similar to that reported previously for glycoprotein carbohydrates(Reference Honda, Yamamoto and Suzuki10, Reference Suzuki, Yamamoto and Kuwahara11).
The minimally glycosylated GMP contained 3·5 (sd 0·1) % N-acetylneuraminic acid and 1·5 (sd 0·1) % galactose, and the glycosylated GMP contained 12·0 (sd 0·3) % N-acetylneuraminic acid and 4·2 (sd 0·2) % galactose.
Subjects and methods
Twenty-two overweight or obese male volunteers (56·3 (sd 8·0) years) were recruited from the Commonwealth Scientific and Industrial Research Organisation (CSIRO) volunteer database.
Volunteers in this database are members of the public who have volunteered for studies at CSIRO and have provided consent to be contacted regarding a variety of studies. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the CSIRO Human Nutrition Human Research Ethics Committee. Written informed consent was obtained from all subjects.
Inclusion criteria were male sex, age 20–65 years and BMI >25 kg/m2, with no recent history (past 3 months) of weight loss or changes to diet or physical activity routine and an unrestrained eater (i.e. restrained eater questionnaire score >10)(Reference Stunkard and Messick12). Exclusion criteria were type 1 or type 2 diabetes or active liver and kidney disease as noted from medical questionnaire, current gastrointestinal disease, past history of gastrointestinal surgery which may affect study outcomes, hypersensitivity to study foods (casein, whey or wheat), medications which affect gastrointestinal motility or hunger/appetite (e.g. metoclopramide, domperidone and cisapride, anticholinergic drugs (e.g. atropine) and erythromycin) or inability to comprehend study protocol. Twenty male volunteers completed the study (56·9 (sd 7·2) years, 97·4 (sd 8·1) kg, BMI 31·5 (sd 3·0) kg/m2). The volunteers in the study had a sedentary lifestyle estimated by a prestudy questionnaire. Two participants withdrew for personal reasons unrelated to the study.
The study was a randomised double-blind design of four different preload compositions with a 3 d interval between study treatments. The preload compositions were controlled for colour, taste and texture, and this was achieved by adding a non-nutritive artificially sweetened chocolate syrup. The nutrient composition of the chocolate syrup (per 70 g) was 95 kJ, 0·4 g protein, 9·7 g carbohydrate and 0·2 g fat. The palatability of the preloads was tested by CSIRO staff before the study. The preloads were served in an opaque container, and were consumed through a straw to limit any effect of appearance or smell on response and palatability. Preload compositions were prepared on the day before the study. The amounts of protein in each of the test products used in the preloads were 41·3 g for the minimally glycosylated GMP fraction, 42·3 g for the glycosylated GMP fraction and 44·4 g for GMP-depleted whey protein concentrate fraction, and total energy intake of the preload compositions was 895 kJ. Volunteers were asked to consume the preload within 5 min of commencing to drink.
A controlled meal was provided to each participant for consumption on the evening before trial commencement. This provided 3020 kJ, 58 g protein, 25 g fat, 56 g carbohydrate and 21 g fibre. Approximately, 25–30 % of the estimated energy requirements were provided by the evening meal. The study participants were asked to abstain from alcohol consumption and to avoid excessive exercise on the evening before visiting the clinic. Volunteers were also asked to record any food eaten on the evening before each clinic visit. A hot lunchtime meal was provided for each participant 3 h after the preload treatment, where they could eat ad libitum until satisfied. Volunteers were given a choice of three options for this lunchtime meal: pasta with bolognaise sauce, veal casserole with rice or chicken curry with rice. In order to control for energy density, volunteers consumed the same meal for all the four study treatments. The amount of food given to each participant, the amount of food consumed and the amount remaining after eating until satisfied were weighed to calculate energy and nutrient intakes. This protocol has been described previously(Reference Bowen, Noakes and Clifton13).
Subjects rated their appetite using validated(Reference Flint, Raben and Blundell14) visual analogue scale before the preload and after every blood sample collection. The visual analogue scale was adapted to a sliding scale, computerised format (Northeast Data Corp. Slider ActiveX Custom Control (1.0) Charlotte, NC, USA)(Reference Bowen, Noakes and Clifton13). The questions were related to hunger, satisfaction, fullness and prospective food intake. A 100-mm horizontal red line was shown below each question, and opposite extremes of the feeling were described at either end of the line. Subjects moved the cursor along the line using the mouse to indicate how they felt at that moment.
A cannula was inserted into a forearm vein, and a fasting blood sample was collected for each participant. The preload was consumed at approximately 09.00 hours, and subsequent blood samples were collected at 15, 30, 60, 90, 120 and 180 min after the consumption of the preload for the measurement of CCK. The cannula was removed after the final blood sample collection. Biochemical analyses for the satiety hormone CCK were performed after study completion on all blood samples that had been taken for each participant. We have described this protocol previously(Reference Bowen, Noakes and Clifton13).
The blood was collected in EDTA (1 g/l) tubes containing aprotinin (5000 KIU (Kallikrein inhibitor units) per 8 ml blood; Mayne Pharma, Melbourne, Australia) and DPP-IV inhibitor (80 μl per 8 ml blood; Australian Laboratory Services) and stored on ice until processed. The plasma was isolated by centrifugation for 10 min at 2000 g, (5°C; Allegra XR-12 Centrifuge) and stored at − 80°C until analysis.
Statistical analysis
Statistical analyses were performed using SPSS 14.0 for WINDOWS (SPSS, Inc., Chicago, IL, USA). Repeated-measures ANOVA was used to assess the effect of time and the effect of treatment on the outcome variables measured, energy intake, CCK and subjective assessment of satiety. Statistical significance was set at P ≤ 0·05. All data are presented as mean values and standard deviations.
Results
Food intake
Energy intake at the hot lunchtime meal was not different between treatments (P = 0·72): 3985 (sd 917) kJ GMP-depleted whey protein concentrate fraction, 3785 (sd 1037) kJ glycosylated GMP fraction, 3797 (sd 1023) kJ minimally glycosylated GMP fraction and 3870 (sd 999) kJ glucose. In addition, there were no differences between treatments for protein (P = 0·71), fat (P = 0·70) or carbohydrate (P = 0·59) intake at the hot lunchtime meal (Table 1).
Table 1 Energy and macronutrient intakes at a hot buffet lunch 3 h after the consumption of preloads containing glycomacropeptide (GMP)-depleted whey protein concentrate (WPC) fraction, glycosylated GMP fraction, minimally glycosylated GMP fraction and glucose*
(Mean values and standard deviations)
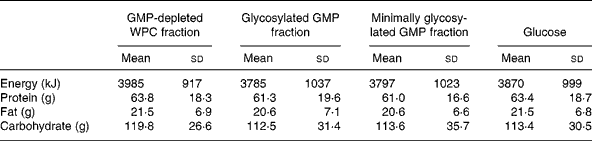
* Data were analysed using repeated-measures ANOVA.
Cholecystokinin
There were no significant differences in CCK concentrations between the preload treatments (P = 0·45; Fig. 1).

Fig. 1 The effect of four preloads containing either glycomacropeptide (GMP)-depleted whey protein concentrate (WPC) fraction, minimally glycosylated GMP fraction, glycosylated GMP fraction or glucose on plasma cholecystokinin (CCK) levels. Data are mean values with their standard errors. Data were analysed using repeated measures ANOVA. There was no time by treatment interaction (P = 0·45). –♦–, GMP-depleted WPC fraction; –■–, glycosylated GMP fraction; –▲–, minimally glycosylated GMP fraction; – × –, glucose.
Visual analogue scale
There was a time-by-treatment effect (P = 0·043) for the visual analogue scale question ‘How satisfied do you feel?’. There were no differences in the visual analogue scale questions ‘How hungry do you feel?’ (P = 1·0), ‘How full do you feel?’ (P = 0·952), ‘How much do you think you can eat?’ (P = 0·531), ‘Would you like to eat something sweet?’ (P = 0·239), ‘Would you like to eat something salty?’ (P = 0·4), ‘Would you like to eat something savoury?’ (P = 0·295) and ‘Would you like to eat something fatty?’ (P = 0·728).
Discussion
The main findings of the present study were that none of the naturally occurring GMP glycoforms had an effect on CCK concentrations, subjective measures of satiety, and energy or macronutrient intake when compared with glucose.
These findings are in contrast to previous studies in which a glucose-containing preload compared with preloads containing a similar amount of protein from whey or casein was consumed(Reference Bowen, Noakes and Trenerry15, Reference Bowen, Noakes and Clifton16). In a study by Bowen et al. (Reference Bowen, Noakes and Trenerry15), nineteen overweight men consumed liquid preloads containing 52 g protein from whey or casein and 56 g lactose or glucose. In contrast to the present study, energy intake was 10 % higher after the consumption of glucose preload compared with lactose and protein preloads (P < 0·05). Also in contrast to the present study, CCK was 71 % higher 90 min after the consumption of protein preloads compared with glucose and lactose (P < 0·05). In a second study by Bowen et al. (Reference Bowen, Noakes and Clifton16), liquid preloads containing 50 g protein from whey, soya and gluten, or 63 g glucose and 1200 kJ were consumed. Once again in contrast to the present study, energy intake was 10 % lower after the consumption of all protein preloads compared with glucose (P < 0·05), and CCK concentrations were elevated after the consumption of protein preloads. It is not clear why differences between the protein and glucose preloads were not observed in the present study as the participants were similar in age and BMI. One reason may be that the protein content of the preloads was 10 g less and the energy was 170 J less in the present study, and differences may have been observed if the lunchtime meal had been offered earlier. The inter-meal interval of 3 h may have been too long to detect subtle differences. Evidence to support this is provided by Bowen et al. (Reference Bowen, Noakes and Clifton16), in which twenty-eight obese men consumed preloads containing 50 g whey, fructose, glucose or 25 g whey+25 g fructose and 1100 kJ. While CCK concentrations were increased after the consumption of protein preload, energy intake was not different between preloads. The inter-meal interval was 4 h in the present study, suggesting that this may have been too long to detect small differences in energy intake. Veldhorst et al. found that energy intake at a lunchtime meal 3 h later was lower after a breakfast meal containing whey than after a breakfast containing whey without GMP, suggesting that GMP may have been responsible for the effect(Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen17). However, the energy content of the test breakfast used was 2520 (sd 70) kJ much greater than that in the present study, in which it was 895 kJ. It may be that hunger from the lower energy load may have overwhelmed any subtle effect of either protein or GMP. The volunteers were also young and lean in the Veldhorst studies. Energy and nutrient intakes from the controlled meal the evening before in the present study may have been less than usual, further contributing to hunger. However, as we did not collect food records before the study, we do not know whether usual food intake was greater than that provided.
In a separate study, Veldhorst et al. (Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen18) found that whey was more satiating than either soya or casein only at the 10 % of energy level and not at the 25 % of energy level. But there were no differences in energy intake at either protein level. In a later study, Veldhorst et al. (Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen19) found that food intake at lunch was 20 % lower 180 min after a breakfast containing α-lactalbumin, gelatin or gelatin plus tryptophan compared with breakfast containing casein, soya, whey and whey with GMP, which were not different from each other.
Similar to the present results, Lam et al. (Reference Lam, Moughan and Awati20) observed that energy and macronutrient intakes were not different following preloads given 30 min before lunch containing maltodextrin, whey protein isolate with no GMP, whey protein isolate with 21 % naturally present GMP or whey protein isolate with 21 % naturally present GMP plus 20 g added GMP. The energy content of the preloads, 1300 kJ, was greater than that in the present study with similar total protein (43–46 g) in the whey-containing preloads. Burton-Freeman(Reference Burton-Freeman8) also found that while satiety scores were greater after the consumption of protein preloads, energy and macronutrient intakes at a test lunch, 1 h after the consumption of preload, were not different between treatments. Preloads contained 1000 kJ energy and whey protein isolate, whey protein without GMP, GMP isolate (0·8 g) or a high carbohydrate control(Reference Burton-Freeman8). Thus, the majority of studies have not shown that whey with GMP or isolated GMP is superior to whey without GMP or indeed any other protein source. The level of GMP fed in the present study was also higher than that fed in any other study, positive or negative. Inconsistent effects of different proteins and peptides have been shown in other studies. Diepvens et al. (Reference Diepvens, Häberer and Westerterp-Plantenga21) observed lower hunger, desire to eat and thirst after consumption of pea compared with milk protein or combined pea and whey proteins(Reference Diepvens, Häberer and Westerterp-Plantenga21). Pea and whey protein separately led to greater satiety and fullness compared with milk protein or the combined pea and whey proteins in overweight subjects given 1024 kJ and 15 g protein. However, despite these differences in subjective measures, there was no effect on energy intake.
It has been postulated that that CCK responses may be abnormal in obesity. Zwirska-Korczala et al. (Reference Zwirska-Korczala, Konturek and Sodowski22) observed that fasting CCK concentrations were lower in morbidly obese individuals compared with lean controls, and that the postprandial CCK response was reduced in morbidly obese individuals(Reference Zwirska-Korczala, Konturek and Sodowski22). However, in a study from our group by Bowen et al. (Reference Bowen, Noakes and Clifton16), there was no difference between lean and obese subjects in fasting CCK concentrations. Additionally, there was no effect of body weight on postprandial CCK. The effect of body weight status on postprandial CCK responses remains to be clarified.
Conclusions
These results suggest that these protein fractions in the dose used were not effective in reducing food intake at a subsequent meal, and that they did not affect postprandial CCK concentrations. The extent of GMP glycosylation had no influence on the results.
Acknowledgements
The present study was funded by the CSIRO Food Futures Research Flagship. The authors have no conflicts of interest in relation to the present work. P. M. C. designed the study and contributed to interpretation of the data and the manuscript. J. B. K. contributed to the study design, managed the clinical trial, analysed the data and drafted the manuscript. B. W. W., C. M. T., F. J. and K. D. contributed to the development of the analysis method, the technology to produce the protein fractions and the manuscript. The help of the clinical research unit staff and laboratory staff at CSIRO Human Nutrition is gratefully acknowledged in the conduct of the study. We also thank the volunteers for participating in the study.




