Porcine circovirus type 2 (PCV2) is a circular, single-stranded DNA virus that was initially associated with a wasting disorder recognised as post-weaning multi-systemic wasting syndrome(Reference Allan, McNeilly and Kennedy1, Reference Ren, Yu and Liu2). However, later studies have indicated that PCV2 is also associated with porcine dermatitis and nephropathy syndrome, reproductive disorder, enteritis, proliferative and necrotising pneumonia and porcine respiratory disease complex, all of which are now called porcine circovirus-associated diseases or porcine circovirus diseases(Reference Segales, Allan and Domingo3, Reference Opriessnig, Meng and Halbur4). These diseases are usually characterised by progressive weight loss, a generalised enlargement of the lymph nodes, and pallor, jaundice and diarrhoea in some cases(Reference Stevenson, Kiupel and Mittal5). In addition to being highly pathogenic, it is found worldwide and therefore imposes a significant economic burden on the swine industry. Thus, many methods have been proposed to control PCV2 infection, such as containment and control, sound husbandry, herd management, good sanitation, immunisation and antibiotic therapy(Reference Jung, Toan and Cho6). Unfortunately, many of these treatments are of limited use(Reference Monroe and Polk7). Therefore, nutritional regulation with functional amino acids to enhance the immune response might be useful as a prophylactic measure against PCV2 infection.
We previously found that dietary supplementation with arginine or glutamine enhanced the immune function in PCV2-infected mice, and may help eliminate PCV2 in experimentally infected mice(Reference Ren, Luo and Wu8–Reference Ren, Li and Yu10). We also found that dietary supplementation with arginine or glutamine partially reversed reproductive failure in mice caused by PCV2 infection(Reference Ren, Luo and Wu8–Reference Ren, Li and Yu10). As with arginine and glutamine, proline plays a versatile role in cell metabolism, physiology, intracellular redox status, oxidative stress and immune function(Reference Liu, Wu and Yin11–Reference Tan Bie, Yin and Liu16). Other reports have indicated that proline concentrations in porcine amniotic fluid increased by 141 % between days 30 and 60 of gestation(Reference Wu, Bazer and Tou17, Reference Wu, Bazer and Datta18). Similarly, concentrations of proline in ovine amniotic fluid increased from 89 to 172 μm, whereas concentrations in ovine allantoic fluid increased by 172 % between days 30 and 60 of pregnancy(Reference Kwon, Spencer and Bazer19). These interesting results suggested that proline may play a vital role in fetal development. However, despite the versatile role of proline, there is little information available regarding the effect of proline on infection. Therefore, the aim of the present study was to determine the effect of dietary supplementation with proline in PCV2-infected pregnant and non-pregnant mice.
Materials and methods
Preparation of porcine circovirus type 2 stock
A PCV2 infectious clone constructed by self-ligation of the PCV2 genome via a SacII enzyme site was used to generate virus stock pools required for experimental infections. Briefly, the continuous porcine kidney cell line PK-15(Reference Yao, Yin and Li20), free of PCV1 and PCV2, was cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 6 % (v/v) fetal calf serum. The cell monolayer was dispersed using trypsin–EDTA and suspended in RPMI-1640 medium supplemented with 6 % (v/v) fetal calf serum. Cells were simultaneously infected with the PCV2 infectious clone. After 72 h of incubation, the infected cells were frozen and thawed three times, and the cell mixture was tested by PCR before being stored at − 20°C. PCV2 stocks were titrated on PK-15 cells(Reference Yao, Yin and Li20).
Experimental protocol
Expt 1
The experiment was conducted as described previously with some modifications(Reference Kong, Yin and Wu12). A total of forty female primiparous KM mice were obtained from the Laboratory Animal Center of Central South University, Hunan, China. The animals were housed in a pathogen-free mouse colony (temperature 20–30°C, relative humidity 45–60 % and lighting cycle 12 h/d) and had free access to food and drinking water. The animals were randomly assigned to a proline group (0·6 % proline+gestation diet, n 20) or a control group (isonitrogenous 0·46 % alanine+gestation diet, n 20). The amino acid content in the gestation diet was measured using the methods of Yin et al. (Reference Yin, Zhang and Huang21). Mice started to mate after 3 d of accommodation. An observable pessus was considered to be a prerequisite for judging copulation. Upon successful copulation, mice were fed diets supplemented with proline or alanine. Overall, nine mice in each group mated successfully. All mice were infected with PCV2 (2000 TCID50) on the 10th day after pregnancy, and the abortion rate was recorded in each group. Blood, serum, organs and the fetus were collected from female mice on the day of delivery for further analysis. A routine blood examination was performed at Huangxing Hospital, Changsha, China. Serum levels of IL-1β, IL-6, TNF-α and C-reactive protein (CRP) were measured using ELISA kits in accordance with the manufacturer's instructions (Cusabio Biotech Company, Limited). The PCV2 virus load in the serum, lung, spleen and fetus was determined using quantitative PCR. Finally, microscopic lesions in the lung, liver and spleen were evaluated in a blinded fashion by a veterinary pathologist.
Expt 2
A total of twenty female KM mice (body weight 18–22 g) were obtained from the Laboratory Animal Center of Central South University, Hunan, China. The animals were housed in a pathogen-free mouse colony (temperature 20–30°C, relative humidity 45–60 % and lighting cycle 12 h/d) and had free access to food and drinking water. The animals were randomly assigned to a proline group (0·6 % proline+gestation diet, n 10) or a control group (isonitrogenous 0·46 % alanine+gestation diet, n 10). After 3 d of accommodation, all mice were infected with PCV2 (2000 TCID50) and fed a diet that was supplemented with either proline or alanine. At 7 d post-infection, all mice were killed to collect blood, serum and organs for further analysis. Blood parameters, serum cytokine profile, PCV2 virus load in the serum, lung and spleen, and microscopic lesions in the lung, liver and spleen were collected as in Expt 1.
All animal experiments were performed according to the guidelines of the Laboratory Animal Ethical Commission of the Chinese Academy of Sciences.
DNA extraction and porcine circovirus type 2 quantitative PCR
DNA was extracted from samples (spleen (5 mg), lung (10 mg), fetus (10 mg) and serum (100 μl)) using Tissue Genomic DNA Extraction Kits (Fisher). DNA from the samples was eluted with 80 μl of elution buffer and stored at − 20°C for further use. DNA extracts were used for the quantification of PCV2 genomes by real-time PCR. Before the quantification of PCV2 genomes in the collected samples, a PCV2 real-time PCR standard was established. Briefly, a PCV2 genome was cloned in the pMD®18-T vector (TaKaRa) after PCR amplification with the following primers: forward 5′-CCGCGGGCTGGCTGAACTTTTGAAAG-3′ and reverse 5′-CCGCGGAAATTTCTGACAAACGTTAC-3′ (GenBank accession no. EU095020), and transformed in TOP10-competent cells (Invitrogen). The plasmid was prepared using a PureLinkTM HiPure Plasmid Midiprep Kit (Invitrogen). PCV2 plasmid was mixed with mouse DNA extracted from a PCV2 PCR-negative blood sample. For PCV2 quantification, 10-fold dilutions of this mixture (from 1011 to 102 PCV2 copy numbers/μl) were used as a standard. PCR was performed using a SYBR Green detection kit (TaKaRa), containing MgCl2, deoxyribonucleotide triphosphate and Hotstar Taq polymerase. Then, 1 μl of template solution was added to a total volume of 10 μl containing a 5 μl SYBR Green mix, and 0·2 μl each of the forward and reverse primers (10 μm). We used the following protocol: (1) predenaturation (30 s at 95°C); (2) amplification and quantification, repeated forty cycles (5 s at 95°C, 34 s at 60°C); (3) melting (60–99°C at a heating rate of 0·1°C/s and fluorescence measurement).
Histopathology
Tissue samples of the spleen, liver and lung from mice that exhibited macroscopic lesions were fixed in 10 % neutral buffered formalin, embedded in paraffin, sectioned (5 mm thick) and stained with haematoxylin and eosin for histopathological examination. Microscopic lesions were evaluated in a blinded fashion by a veterinary pathologist using a previously described scoring system(Reference Opriessnig, Thacker and Yu22). Lung sections were examined for the presence and severity of interstitial pneumonia, and scored on a scale from 0 (normal) to 6 (severe diffuse). Sections of the liver were evaluated for the presence of lymphohistiocytic inflammation, and scored on a scale from 0 (none) to 3 (severe). The spleen was evaluated for the presence of lymphoid depletion and histiocytic inflammation, and scored on a scale from 0 (normal) to 3 (severe).
Statistical analysis
All statistical analyses were performed using SPSS 16.0 software. Group comparisons were performed using Student's t test. Data on the abortion rates of pregnant mice and the detection rates of PCV2 virus in samples were analysed by χ2 analysis. Differences were considered as significant at P< 0·05. Data are expressed as means with their standard errors of the mean.
Results
Clinical observation
As observed in our previous study, abortion occurred in late pregnancy. Overall, the abortion rates in the control and proline groups were 44·44 % (n 4/9) and 22·22 % (n 2/9), respectively. However, dietary supplementation with proline had no effect (P>0·05) on abortion rates in PCV2-infected pregnant mice, although a numerically lower abortion rate was observed compared with the control. Meanwhile, there was no difference in litter size between the proline and control groups (10·5 (sem 0·43) v. 10·8 (sem 0·73)).
Cytokine profile and blood examination
As shown in Table 1, dietary supplementation with proline significantly (P= 0·03) increased the serum CRP level, whereas there were no differences (P>0·05) in serum IL-1β, IL-6 and TNF-α levels in PCV2-infected pregnant mice. In PCV2-infected non-pregnant mice, the serum TNF-α level in the proline group was significantly (P= 0·01) higher than that in the control alanine group (Table 2). In the pregnant mouse model, there were no significant differences in serum IL-1β, IL-6 and CRP levels between the proline and control alanine groups (Table 2). Furthermore, in PCV2-infected pregnant mice, no important difference in blood parameters was found between the proline and control groups (Table 3). In contrast, in PCV2-infected non-pregnant mice, dietary supplementation with proline significantly (P< 0·05) increased leucocytes, lymphocytes and neutrophilic granulocytes, compared with dietary supplementation with alanine (Table 4).
Table 1 Effects of dietary supplementation with proline on serum cytokine profile in porcine circovirus type 2-infected pregnant mice* (Mean values with their standard errors)
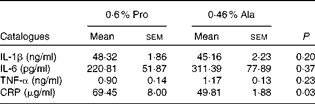
Pro, mice fed with a normal gestation diet with 0·6 % proline supplementation; Ala, mice fed with a normal gestation diet with 0·46 % alanine supplementation, as the isonitrogenous control; CRP, C-reactive protein.
* Five mice from the Pro group and six mice from the Ala group were used for serum cytokine analysis.
Table 2 Effects of dietary supplementation with proline on serum cytokine profile in porcine circovirus type 2-infected non-pregnant mice* (Mean values with their standard errors)
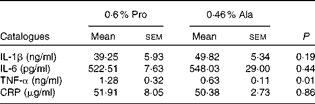
Pro, mice fed with a normal gestation diet with 0·6 % proline supplementation; Ala, mice fed with a normal gestation diet with 0·46 % alanine supplementation, as the isonitrogenous control; CRP, C-reactive protein.
* Six mice from the Pro group and six mice from the Ala group were used for serum cytokine analysis.
Table 3 Results of a routine blood examination in porcine circovirus type 2-infected pregnant mice (n 6) (Mean values with their standard errors)
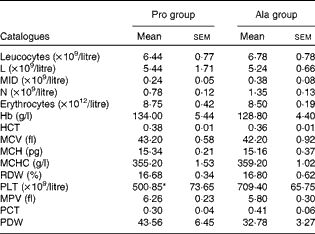
Pro, mice fed with a normal gestation diet with 0·6 % proline supplementation; Ala, mice fed with a normal gestation diet with 0·46 % alanine supplementation, as the isonitrogenous control; L, lymphocytes; MID, intermediate cell; N, neutrophilic granulocyte; HCT, haematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular Hb, MCHC, mean corpuscular Hb concentration; RDW, red cell distribution width; PLT, platelet; MPV, mean platelet volume; PCT, thrombocytocrit; PDW, platelet distribution width.
* Mean value was significantly different with respect to the control group (P< 0·05).
Table 4 Results of a routine blood examination in porcine circovirus type 2-infected non-pregnant mice (Mean values with their standard errors)
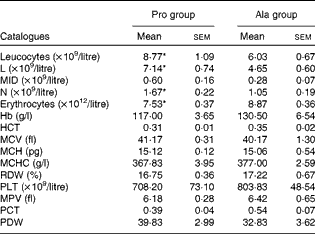
Pro, mice fed with a normal gestation diet with 0·6 % proline supplementation; Ala, mice fed with a normal gestation diet with 0·46 % alanine supplementation, as the isonitrogenous control; L, lymphocytes; MID, intermediate cell; N, neutrophilic granulocyte; HCT, haematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular Hb; MCHC, mean corpuscular Hb concentration; RDW, red cell distribution width; PLT, platelet; MPV, mean platelet volume; PCT, thrombocytocrit; PDW, platelet distribution width.
* Mean values were significantly different with respect to the control group (P< 0·05).
Porcine circovirus type 2 virus load and microscopic lesions
As shown in Table 5, in PCV2-infected pregnant mice, PCV2 DNA was detected in serum (n 3/6), lung (n 5/6), fetus (n 0/6) and spleen (n 4/6) in the alanine group, whereas it was detected in serum (n 4/6) in the proline group. In the alanine group, the mean PCV2 log10 genomic copies per ml (and/or per g) was 6·36 (sem 1·42) for the spleen, 8·14 (sem 1·89) for the lung and 3·72 (sem 0·25) for the serum, whereas in the proline group, this was 3·21 (sem 0·28) for the serum. Meanwhile, in the lung, the PCV2 virus detection rate in the alanine group was significantly (P= 0·015) higher than that in the proline group, whereas no difference was found for the other organisms. Similarly, as shown in Table 6, in PCV2-infected non-pregnant mice, PCV2 DNA was detected in serum (n 1/6) and lung (n 3/6) in the alanine group, but found only in serum (n 1/6) in the proline group, and the mean PCV2 log10 genomic copies per ml (and/or per g) was 3·39 (sem 0·05) for the lung and 3·27 for the serum in the alanine group, while in the proline group, this was 3·62 for the serum. No difference was found for the PCV2 virus detection rates between the alanine and proline groups.
Table 5 Porcine circovirus type 2 (PCV2) virus load in the serum, fetus, spleen and lung of PCV2-infected pregnant mice† (Mean values with their standard errors)
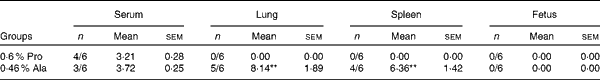
Pro, mice fed with a normal gestation diet with 0·6 % proline supplementation; Ala, mice fed with a normal gestation diet with 0·46 % alanine supplementation, as the isonitrogenous control.
** Mean values were significantly different with respect to the proline group (P< 0·01).
† Five mice from the Pro group and six mice from the Ala group were used for PCV2 virus load analysis. The detection rate is shown as number of positive mice/number of tested mice. The PCV2 load is shown as mean PCV2 log10 genomic copies per ml (and/or per g).
Table 6 Porcine circovirus type 2 (PCV2) virus load in the serum, spleen and lung of PCV2-infected non-pregnant mice† (Mean values with their standard errors)
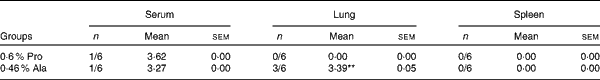
Pro, mice fed with a normal gestation diet with 0·6 % proline supplementation; Ala, mice fed with a normal gestation diet with 0·46 % alanine supplementation, as the isonitrogenous control.
** Mean value was significantly different with respect to the proline group (P< 0·01).
† Six mice from the Pro group and six mice from the Ala group were used for PCV2 virus load analysis. The detection rate is shown as number of positive mice/number of tested mice. The PCV2 load is shown as mean PCV2 log10 genomic copies per ml (and/or per g).
As observed in previous reports and observations, PCV2 infection was associated with interstitial pneumonia and alveolar wall thickening due to macrophages and lymphocytes in the lung, lymphohistiocytic inflammation in the liver, and lymphoid depletion and histiocytic inflammation in the spleen(Reference Jung, Toan and Cho6, Reference Opriessnig, Thacker and Yu22, Reference Opriessnig, Patterson and Meng23). In both PCV2-infected pregnant and non-pregnant mice, the lesions in the lung in the proline group (Fig. 1(a)) were not as severe as those in the control group (Fig. 1(b)). The proline group (Fig. 1(c)) showed less lymphohistiocytic inflammation in the liver than the alanine group (Fig. 1(d)). Furthermore, dietary supplementation with proline significantly decreased microscopic lesions in the spleen (Fig. 1(e)v. (f)). The microscopic lesion scores in the examined tissue samples are shown in Table 7. Mice in the dietary proline group showed a significant decrease in microscopic lesion scores in the lung, liver and spleen compared with those in the alanine group (P< 0·01), although the difference in the lung was not statistically significant in non-pregnant mice (P= 0·072).

Fig. 1 Histopathological findings in the lung, liver and spleen ( × 100). Mild interstitial pneumonia was evident in the lungs in the proline group (a) compared with the control group (b). The proline group (c) showed less lymphohistiocytic inflammation in the liver than the alanine control group (d). Additionally, the proline group showed less severe lymphoid depletion (e) in the spleen compared with the control group (f). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Table 7 Microscopic lesion scores for the tissue samples in porcine circovirus type 2 (PCV2)-infected pregnant and non-pregnant mice* (Mean values with their standard errors)
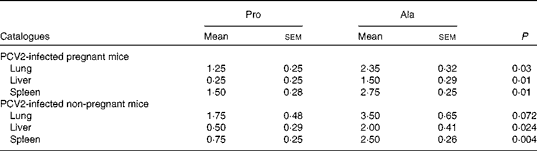
Pro, mice fed with a normal gestation diet with 0·6 % proline supplementation; Ala, mice fed with a normal gestation diet with 0·46 % alanine supplementation, as the isonitrogenous control.
* In pregnant mice, five mice from the Pro group and six mice from the Ala group were used for microscopic lesion analysis. In non-pregnant mice, six mice from the Pro group and six mice from the Ala group were used for microscopic lesion analysis.
Discussion
Among the numerous prophylactic measures that are currently available, nutritional regulation offers several advantages. The most compelling advantage is that it can increase resistance to a group of pathogens or even all pathogens, and thus can significantly reduce the incidence of diseases and the shedding of pathogens. Similar to other functional amino acids, proline plays an important role in cellular redox, cell proliferation, immunity and other physiological phenomena(Reference Li, Yin and Li13, Reference Hu, Phang and Valle14, Reference Wu, Bazer and Datta18). Unfortunately, there is currently a belief that proline is not especially necessary for gestating, neonatal, growing, infected or sick animals(Reference Elango, Ball and Pencharz24). This unfavourable opinion reflects a lack of knowledge regarding the role of proline in immune regulation in infectious and morbid mammals. Thus, the present study was performed to examine the role of dietary supplementation with proline in PCV2-infected pregnant and non-pregnant mice. The dose of proline used in dietary supplementation was selected according to our previous study (WK Ren et al., unpublished results) and many Chinese reports. Pregnant and non-pregnant mouse models were chosen to perfect our experimental protocol and to enhance the creditability of the present experimental results.
PCV2 has been associated with many disease syndromes in pigs including swine reproductive failure, since it can replicate in embryos, lead to embryonic death and be passed through vertical transmission(Reference Mateusen, Sanchez and Van Soom25, Reference Mateusen, Maes and Van Soom26). Similarly, a significant abortion rate was observed in pregnant mice after PCV2 infection at 0–14 d of gestation(Reference Ren, Li and Yu10). In the present study, dietary supplementation with proline had no effect on abortion rates in PCV2-infected pregnant mice. However, a numerically higher abortion rate was observed for the control compared with proline-supplemented mice, thus suggesting a need for further research with a larger sample size to adequately examine any potential role that dietary supplemental proline might have on abortion rates in PCV2-infected mice. However, the serum CRP level in the proline group is significantly higher than that in the control. CRP plays a role in host defence against bacterial pathogens, protection from lethal bacterial infection and endotoxaemia, the activation of complement, opsonisation and induction of phagocytosis(Reference Szalai, Briles and Volanakis27–Reference Szalai30). Thus, this interesting observation indicates that proline supplementation confers a beneficial role in PCV2 infection. Another finding that dietary supplementation with proline significantly decreases both the PCV2 virus load and microscopic lesions in tissues also supports this conclusion. Collectively, these compelling results suggest that dietary supplementation with proline confers a significant positive effect in PCV2-infected pregnant mice.
PCV2 infection has been associated with lymphocyte depletion, interference with antigen presentation, apoptosis of monocyte/macrophage lineage cells and other antigen-presenting cells, and inhibition of the activity of natural interferon-producing cells(Reference Kennedy, Moffett and McNeilly31–Reference Vincent, Balmelli and Meehan33). Thus, PCV2 infection has been referred to as an immunosuppressant disease. In the present PCV2-infected non-pregnant mice, dietary supplementation with proline significantly increased leucocytes, lymphocytes and neutrophilic granulocytes in the blood. TNF-α, which is secreted by both macrophages and monocytes, is an important member of the TNF family that plays a key role in immune regulation, and enhances lymphoid development, cell proliferation, differentiation, activation and death(Reference Smyth and Johnstone34, Reference Ch'en, Xu and Liu35). The serum TNF-α level in the proline group is much higher than that in the control alanine group. Moreover, we also found that interstitial pneumonia in the lung, lymphohistiocytic inflammation in the liver and lymphoid depletion in the spleen are more severe in the alanine group than in the proline group. Furthermore, dietary supplementation with proline decreased the PCV2 virus load in the lung. Taken together, these findings suggest that supplementation with proline also had a beneficial effect in PCV2-infected non-pregnant mice.
Similar to arginine and glutamine, proline plays an important role in immune responses. Duval et al. (Reference Duval, Demangel and Munier-Jolain36) reported that proline plays a role in protecting lymphocytes from apoptosis, stimulating cell growth and promoting antibody production. Consistently, other studies have also shown that proline is crucial for wound healing and injury recovery mediated by cells of the immune system. Studies have found that proline plays an important role in multiple biochemical and physiological processes in human subjects and animals as a signalling molecule, a sensor of cellular energy status and a source of pyrroline-5-carboxylate and superoxide anion(Reference Phang, Donald and Pandhare37–Reference Phang, Liu and Zabirnyk39). Proline also participates in cell differentiation, as well as conceptus growth and development(Reference Wu, Bazer and Datta18). Furthermore, proline is a major nitrogenous substrate for the synthesis of polyamines in the small intestine and placenta, which expands its role in fetal and neonatal nutrition(Reference Wu, Bazer and Datta18, Reference Wu, Flynn and Knabe40, Reference Wu, Bazer and Hu41). Finally, proline can scavenge free radicals, and thus has vital antioxidant properties(Reference Kaul, Sharma and Mehta42). Consistent with these findings, another promising study has found that proline plays a role in cell growth and function by regulating the mammalian target of rapamycin activation pathway(Reference van Meijl, Popeijus and Mensink43).
In addition to proline itself, proline oxidase also plays a vital role in immune function. For example, Ha et al. (Reference Ha, Oh and Bae44) reported that immune function in the gut is impaired because of a deficiency of intestinal proline oxidase, and thus suggested that proline oxidase may play an important role in immunity. H2O2, a major product of proline oxidation, is a signalling molecule and a cytotoxic agent towards pathogenic bacteria(Reference Shi, Meininger and Haynes45). Wu et al. (Reference Wu, Bazer and Hu41, Reference Wu46) proposed that a high activity of proline oxidase in the porcine placenta and the small intestine of piglets may play a crucial role in protecting these organs from infection during the critical periods of fetal and neonatal development. Sun et al. (Reference Sun, Nonobe and Kobayashi47) indicated that proline oxidase is present in milk and may aid in protecting the neonatal intestine from bacterial and viral challenge(Reference Field48).
In conclusion, dietary supplementation with proline had no effect on abortion rate in pregnant mice infected with PCV2. However, a numerically lower abortion rate was observed for mice fed the proline-supplemented diet compared with the control. Feeding the proline-supplemented diet showed beneficial effects in pregnant and non-pregnant mice in terms of improved blood parameters and cytokine profile, and decreased tissue lesions and the PCV2 virus load in tissues. These benefits and the observed numerical difference in abortion rate warrant further research with sufficient observations to better substantiate any role that proline supplementation might have on abortion rates in PCV2-infected mice, and to elucidate the underlying mechanisms.
Acknowledgements
The present study was jointly supported by the National Basic Research Program of China (2012CB124704), the National Natural Science Foundation of China (no. 31110103909, 30901040, 30928018 and 31101729) and the National Scientific and Technology Support Project (2011BAD26B002-5). Y. Y. and W. R. were in charge of the whole trial. W. R., R. H., Y. L. and X. Y. conducted the animal experiment and wrote the manuscript. M. W., W. L. and T. L. assisted with the animal trial and chemical analyses. The authors have no conflicts of interest to declare.










