A diet rich in whole grains (WG) is widely considered to be beneficial; however, WG consumed as breakfast cereals are reported to have a number of additional benefits, including regulation of body weight, decreased risk of type 2 diabetes and an inverse relationship with CVD-specific and total mortality(Reference Kochar, Djousse and Gaziano1–Reference Fung, Hu and Pereira3). The gut microbiota is increasingly recognised as a co-evolved microbial partner, complementing and extending human-encoded metabolic capabilities and interacting closely with human diet to mediate host health and disease(Reference Jacobs, Gaudier and van Duynhoven4–Reference Tuohy, Gougoulias and Shen7). Supplementation of the gut with fermentable carbohydrates such as inulin and resistant starch has been shown to decrease the risk of chronic disease, and can be partly attributed to modulation of the gut microbiota via the prebiotic effect(Reference Topping, Fukushima and Bird8–Reference Roberfroid11).
Commercially established prebiotics are often non-digestible oligosaccharides such as fructo-oligosaccharides, inulin and oligofructose, together with emerging prebiotics such as xylo-oligosaccharides and arabinoxylan oligosaccharides. Despite a good safety record in large doses, they can be responsible for gastrointestinal discomfort as tolerance, and side effects can vary widely between individuals(Reference Mussatto and Mancilha12). An alternative approach is to use foods that are naturally WG rich which when fermented in vivo possess inherent potential prebiotic activity, as was recently reported by Costabile et al. (Reference Costabile, Klinder and Fava13), during a human volunteer study. It is clear that WG contain a complex mix of bioactive components; however, more research is needed to determine the impact of novel WG sources on the gut microbiota. Therefore, in the present study, the main objective was to determine the prebiotic potential of a breakfast cereal enriched with WG derived from maize (WGM). A secondary objective was to determine the impact of WGM on colonic metabolic output (SCFA), bowel habit and on the fasted lipid profile.
Materials and methods
Subjects
Potential volunteers were selected using an initial telephone interview followed by a screening appointment (to collect anthropometric measures and a fasted blood sample). Volunteers were excluded for the following reasons: pregnancy/lactating, participation in another prebiotic study within 3 months, antibiotic administration (within the previous 6 months), anaemia, hyperlipidaemia or chronic gastrointestinal complaints. Any intake of other specific drugs active on gastrointestinal motility, or a laxative of any class, for 4 weeks before the study was all prohibited.
Thirty-three healthy volunteers were recruited onto the study with one withdrawal due to personal reasons (n 32). The study group consisted of eleven males and twenty-one females; age range 20–51 years (mean age 31·6 (sd 8) years) and BMI 20–30 (kg/m2). The present study was conducted according to the guidelines laid down in the declaration of Helsinki, and all procedures involving human subjects were approved by the University of Reading research and ethics committee. Written informed consent for participation in the study and for taking of blood samples was obtained from all subjects.
Requirements for diet and medication during the study
During the study, volunteers were not permitted to consume prebiotic supplements (such as inulin or oligofructose), probiotics (e.g. live yoghurts and fermented milk drinks) or WG breakfast cereals (other than that provided by the study).
Study design
The supplementation study was performed in a double-blind, randomised, placebo-controlled crossover manner. For a 2-week period before the intervention, volunteers followed a 2-week restriction diet, which included no consumption of any pre- or probiotics. Volunteers were randomly allocated into one of two groups, one group (n 16) initially consumed breakfast cereal (WGM; 48 g/d) for 3 weeks, followed by a 3-week wash-out period; they then consumed the placebo cereal (non-whole grain (NWG)) (48 g/d) for 3 weeks followed by a final wash-out period. The other group (n 16) followed the same procedure in reverse order. Volunteers were asked to keep daily diaries during the study to record stool frequency, consistency (constipation, hard, formed, soft or diarrhoea), abdominal pain (none, mild, moderate or severe), intestinal bloating (none, mild, moderate or severe) and flatulence (none, mild, moderate or severe).
Test products
The cereals used are commercially available using standard ingredients approved for food use, and were packaged, labelled and randomised by the Cereal Partners Worldwide (Welwyn Garden City, Herts, UK) before the study; therefore, the two products (WGM and NWG) were indistinguishable in terms of colour, texture, taste and smell. The test cereal (WGM) was supplemented with WG derived from maize semolina (to a final concentration of 29·6 %), and contained WG maize cereal grains, sugar, high fructose maize syrup, fat-reduced cocoa powder, wheat starch, maize starch, palm oil, iodised salt (raising agent, dicalcium phosphate and sodium bicarbonate; colour, caramel; artificial flavour, vanilla flavour) and trisodium phosphate. The nutrient composition (per 48 g serving without milk) of WGM provided 1602·5 kJ (383 kcal), 37·04 g total carbohydrate (of which 18·2 g sugars), 2·09 g protein, 1·95 g total fat (of which 0·72 g saturated, 0·68 g monounsaturated and 0·55 g polyunsaturated fat) and total fibre 14·2 g.
Control cereal (NWG) composition was sugar, maize semolina, high fructose maize syrup, palm oil, fat-reduced cocoa powder, wheat starch, maize starch, iodised salt (colour, caramel; artificial flavour, vanilla), sodium bicarbonate and trisodium phosphate. Control cereal (NWG) contained 1640·1 kJ (392 kcal), 39·09 g total carbohydrate (of which 20·95 g sugars), 1·63 g protein, 1·68 g total fat (of which 0·72 g saturated, 0·68 g monounsaturated and 0·27 g polyunsaturated fat) and total fibre 0·81 g.
It also contained 86 g carbohydrates (of which consisted 46·1 g sugars), 3·6 g protein, 3·7 g total fat (containing 1·6 g saturated, 1·5 g monounsaturated and 0·6 g polyunsaturated fat) and 1·8 g fibre.
Enumeration of faecal bacteriology
Freshly voided faecal samples were stored in an anaerobic cabinet (10 % H2, 10 % CO2 and 80 % N2) for no longer than 2 h before processing. Samples were diluted 1 in 10 (w/w) with PBS (0·1 M; pH 7·0) and mixed in a Stomacher 400 (Seward, Norfolk, UK) for 2 min at normal speed.
Faecal bacterial populations were determined using fluorescence in situ hybridisation with molecular probes targeting 16S rRNA sequences. Cy3-labelled oligonucleotide probes were obtained from Sigma Genosys (Sigma Aldrich, Haverhill, Suffolk, UK). Fluorescence in situ hybridisation analysis was performed as described(Reference Napolitano, Costabile and Martin-Pelaez14). The probes used were Bif164, Lab158, Chis150, Eub338 I/II/III, Bac303, Erec482, REC and Ato291 specific for bifidobacteria, lactobacilli/enterococci, clostridia (Clostridium perfringens/histolyticum subgroup), total bacteria, bacteroides groups, members of Clostridium cluster XIVa, Roseburia/Eubacterium rectale group (a component of cluster XIVa) and Atopobium spp., respectively.
SCFA analysis
Aliquots (1 ml) of 10 % (w/v) faecal suspension were dispensed into 1·5 ml eppendorf tubes and then centrifuged (13 000 g for 5 min) to pellet bacteria and other solids. Samples taken from the batch culture vessels or human subjects were centrifuged at 13 000 g for 5 min to remove all particulate matter. Supernatants were then filtered using 0·2 μm polycarbonate syringe filters (Whatman, Maidstone, Kent, UK) and injected (20 μl) into an HPLC system (Merck, Whitehouse Station, NJ, USA) equipped with refractive index detection. The column used was an ion-exclusion REZEX-ROA organic acid column (Phenomenex, Inc., Torrance, CA, USA) maintained at 85°C. H2SO4 in HPLC-grade H2O (0·0025 mmol/l) was used as the eluent, and the flow rate was maintained at 0·5 ml/min. Quantification of the samples was obtained through calibration curves of lactic, acetic, propionic, butyric and valeric acids and branched-chain fatty acids in concentrations ranging between 2·5 and 100 mm.
Blood sample collection
For each volunteer, blood samples were collected into two 10 ml BD SST™ serum separation tubes (BD Vacutainer®; Becton Dickinson, Cowley, Oxon, UK) for the analysis of fasting levels of TAG, glucose, total cholesterol and HDL-cholesterol. Serum was obtained after centrifugation at 1700 g for 10 min, dispensed into 1·5 ml microcentrifuge tubes and stored at − 20°C within 1 h from the collection. For analysis, serum was defrosted on a roller mixer and centrifuged for 5 min at 1500 g.
Determination of plasma TAG, total cholesterol, glucose and HDL-cholesterol
Plasma TAG, glucose, total cholesterol and HDL-cholesterol concentrations were determined using the ILAB 600 clinical chemistry analyser (Instrumentation Laboratories Limited, Cheshire, UK). Test kits (IL test TAG, IL test cholesterol, IL test HDL-cholesterol and IL test glucose (hexokinase)) supplied by Instrumentation Laboratories were used according to instructions for the determination of plasma TAG, total cholesterol, HDL-cholesterol and glucose, respectively. Two quality control samples, Wako control serum I and Wako control serum II (Alpha Laboratories Limited, Eastleigh, Hants, UK), containing normal and abnormal known concentrations of TAG, total cholesterol, HDL-cholesterol and glucose were included at the beginning and at the end of each batch analysis. Sample results were accepted if quality control values were within the range specified by the manufacturers.
Statistics
Results are expressed as a group mean (where n 32) with sd or sem. Data were checked for distribution and variance, and were necessarily transformed before statistical analysis was done using Minitab version 14. Faecal bacterial levels, blood lipids and glucose, faecal SCFA, anthropometric measurements and bowel diary habit data were analysed using general linear model ANOVA with Tukey's post hoc comparison tests where appropriate.
Results
The predominant bacterial groups within the faecal microbiota were enumerated using fluorescence in situ hybridisation (shown in Table 1). After consumption of WGM for 21 d, a significant increase in bifidobacteria (log10 colony-forming unit/g) was observed compared with the respective baseline (P = 0·001). A non-significant increase was also observed after consumption of the control cereal compared with baseline (P = 0·2998). Comparison between baseline levels of both treatment groups showed no significant difference (P = 0·9535); however, comparing both post-treatment groups showed borderline significance (P = 0·0561). Treatment effects were not sustained after 21-d post-supplementation, as a significant reduction was seen when comparing between WGM and final wash-out periods (P = 0·0001).
Table 1 Faecal bacteriology (mean log10 bacteria/g wet weight faeces) enumerated using fluorescence in situ hybridisation during a human intervention study (n 32) following consumption of a breakfast cereal (48 g/d) containing whole grain derived from maize (WGM) or an equivalent control cereal (non-whole grain NWG (placebo))*
(Mean values with standard deviations)
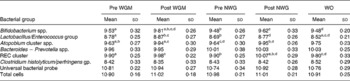
NWG, non-whole grain (placebo); Pre WG, baseline whole grain; post WG, post whole grain; WO, final wash-out period; REC, Ruminococcus–Eubacterium–Clostridium cluster.
a,b,c,d Mean values within a row with superscript letters in common were significantly different (P < 0·05, general linear model ANOVA with Tukey's post hoc test).
* Stool samples were collected on days 0, 21, 42, 63 and 84 (representing baseline sample, and after both treatments and WO).
At baseline, there was no significant difference in the numbers of Lactobacillus/Enterococcus (P = 0·6393); however, during the intervention, lactobacilli levels increased comparably in both WGC and control arms compared with the respective baseline levels, but neither of them was statistically significant (P = 0·6455 for WGC and P = 0·7709 for placebo). The numbers of lactobacilli decreased significantly log10 8·52 (sd 0·21) during final washout compared with both cereal WGC (P = 0·0011) and control (P = 0·0024) arms. With respect to baseline, a similar increase in faecal Atopobium levels was observed after both WGM (P = 0·004) and placebo (P = 0·0002). No significant changes in the number of Bacteroides spp., C. histolyticum subgroup, C. perfringens/histolyticum subgroup and total bacterial numbers (Eubacteria and 4′, 6′-diamidino-2-phenylindole) were observed during the course of the trial. Faecal SCFA, bowel habit data, fasted lipids/glucose and anthropometric measures (blood pressure, BMI and waist circumference) showed no significant changes according to treatment or wash-out periods (P>0·05, data not shown).
Discussion
Recent findings from our group have shown for the first time that consumption of a minimally processed source of WG in the form of a breakfast cereal had a prebiotic effect compared with a wheat bran cereal within a mixed diet, healthy, normolipaemic cohort(Reference Costabile, Klinder and Fava13). The present study builds on the findings in several ways as follows: firstly, the source of WG is from maize instead of wheat; secondly, the cereals are very closely nutritionally matched (apart from the fibre content); and thirdly, we aimed to assess whether a relatively low WG content can still maintain a prebiotic effect, despite the presence of a mixed habitual diet in the study group.
The consumption of WGM resulted in a significant mean increase in faecal bifidobacteria, which is considered a positive indicator of prebiotic activity. To date, this is the first time that breakfast cereal enriched with maize as a WG source has been reported from in vivo data to be potentially prebiotic; it has been suggested that supplementation with pro- or prebiotics may not have the same magnitude of effect between different individuals, and perhaps the levels of indigenous bifidobacteria (or other probiotic strains) present are more important than the doses provided during traditional supplementation(Reference Roberfroid, Van Loo and Gibson15). Indeed, within the present study, volunteers who were most responsive in terms of a bifidogenic effect to the WG cereal had the lowest initial populations of bifidobacteria; conversely, the individuals with the highest initial samples had a less marked response (data not shown). Little or no change was observed in the numbers of total bacteria, Bacteroides spp., C. histolyticum/perfringens group and Atobacterium spp. present in faecal samples collected over the course of the trial which is in agreement with that reported in the literature(Reference Kolida, Tuohy and Gibson16–Reference Tuohy, Kolida and Lustenberger19).
Traditionally, prebiotic activity seems to have been more commonly established using refined fractions or carbohydrates. However, with the current emphasis on the use of dietary strategies to achieve health benefits, it is becoming increasingly important to establish the prebiotic activity of whole foods. However, since we do not as yet know all the components of these foods responsible for these observed prebiotic effects, such foods need to be tested on a case-by-case basis in human feeding studies to determine their impact on the gut microbiota and prebiotic potential. Recent findings have suggested that the protective effects of WG are increased in a composite and unrefined food than that offered by the individual bioactive components(Reference Slavin20, Reference Slavin21). It is clear from different sources of evidence that WG foods are beneficial and act via different mechanisms, including effects mediated by the large intestine, regulation of postprandial glucose and antioxidant effects. These are influenced by processing conditions, the cereal grains used and subject characteristics; therefore, in summary, the present study showed a prebiotic effect from a WG maize cereal, which resulted in a beneficial shift in the faecal microbiota that could provide additional evidence for the health benefits of WG.
Acknowledgements
The authors would like to thank Mrs Jan Luff for helping with recruitment and clinical visit management. The work was generously supported by Cereals Partners Worldwide. The authors made the following contributions to the study: A. L. C.-W., C. L., F. T., B. M., K. G. J. and K. M. T. did the study concept and design, analysis and interpretation of data, preparation of the manuscript; C. M., K. H. and C. N. did the clinical sample processing, sample analysis and acquisition of data. A. L. C.-W., K. G. J., K. M. T., C. M., K. H. and C. N. have no conflict of interest. C. L., B. M. and F. T. are employees of Cereal Partners UK.



