Background and rationale
During the past decade, detrimental health implications of trans-fatty acids (TFA) have been extensively studied, particularly in the context of CVD risk. Increased TFA consumption from ‘industrial’ (iTFA) origin (i.e. partially hydrogenated vegetable oils) has been shown to be positively associated with increased CHD incidence via various mechanisms pertaining to lipid metabolism, insulin sensitivity and inflammation (reviewed in Mozaffarian et al. (Reference Mozaffarian, Aro and Willett1) and Brouwer et al. (Reference Brouwer, Wanders and Katan2)). Emerging epidemiological data have also associated iTFA consumption with an increased risk and/or incidence of breast cancer(Reference Voorrips, Brants and Kardinaal3), prostate cancer(Reference Chavarro, Stampfer and Campos4) and colorectal cancer(Reference Vinikoor, Millikan and Satia5). Consequently, the public has been alerted to restrict the consumption of TFA-containing foods; moreover, TFA content has become a mandatory section on food labels in North America, some European countries and others. For example, Health Canada currently recommends a TFA limit of 5 % of total fat in all products sold to consumers and 2 % for commercial margarines and spreads. Denmark has legislated the content of iTFA to be less than 2 % of total fat in all oil and fat sold separately or as food ingredients. Notably in 2006, these regulatory bodies agreed to endorse the Codex Alimentarius definition of TFA, with an intent to encourage countries to adopt prudent TFA nutrition labelling and TFA food-related policies.
The Codex definition and differences between ruminant and industrial trans-fatty acids
According to the Codex definition, TFA is defined as ‘all the geometrical isomers of monounsaturated and polyunsaturated fatty acids having non-conjugated, interrupted by at least one methylene group, carbon–carbon double bonds in the trans-configuration’, which excludes all isomers in the family of conjugated linoleic acid (CLA). Such exclusion was established from growing literature suggesting potent body-weight reduction and anti-atherogenic properties of CLA, primarily from cell-culture and animal studies(Reference Stachowska6–Reference Reynolds and Roche8). However, a major confusion exists among consumers and industrial bodies to whether or not all trans-fats from ruminant sources are equally detrimental to health and should also be eliminated from the diet. Currently, the trans-fat content on many food labels (and in legislative documents) does not include ruminant CLA isomers, implying it to have differential properties. We wish to point out that other ruminant fatty acids with one or more trans double bonds far more abundant than CLA can remain included on food labels. Indeed, evidence from both epidemiological studies and preclinical experimental models collectively demonstrates the neutral or beneficial health effect of TFA derived from ruminant fat at normal consumption levels(Reference Gebauer, Chardigny and Jakobsen9). As highlighted in a recent quantitative review of prospective cohort studies by Bendsen et al. (Reference Bendsen, Christensen and Bartels10), dietary consumption of ruminant trans-fat may be protective against total as well as fatal CHD events. Very recently, Brouwer et al. (Reference Brouwer, Wanders and Katan2) updated their previous quantitative review to include new studies and adjustments in data analysis(Reference Brouwer, Wanders and Katan11). Consistent with Bendsen et al. (Reference Bendsen, Christensen and Bartels10), ruminant-derived TFA (rTFA) were found to have no adverse effect on biomarkers for CVD at amounts likely to be consumed in the general population (between 2 and 4 g/d)(Reference Brouwer, Wanders and Katan11). Nevertheless, the distinctive health effects of TFA from different food sources (i.e. industrial v. ruminant) have not been clarified in the Codex TFA definition.
Issues surrounding supplemental conjugated linoleic acid
The discovery of weight loss as well as other potential health properties of CLA has been the premise for the commercialisation of CLA supplements for weight management. In European countries, CLA has been approved as a novel food ingredient at a dose of 3 g/d up to 6 months(12); the US Food and Drug Administration has also issued ‘Generally Recognized As Safe’ notifications on similar CLA products for use in specific foods including meal replacement beverages, milk products and fruit juices at 1·5 g CLA/serving and up to 3 g/d(13). However, the efficacy associated with its health claims for all populations remains debated(14). Most recently, concerns have surfaced suggesting the potential adverse effect on atherogenic cholesterol profile from supplemental CLA use in select population groups(Reference Brouwer, Wanders and Katan2, Reference Riserus, Arner and Brismar15–Reference Riserus, Vessby and Arner17). As a result, the Food Standards Australia and New Zealand (FSANZ) proposed to re-evaluate their perception regarding the exclusion of CLA from the TFA definition.
Variations in the interpretation of the Codex definition of trans-fatty acids
It is important to appreciate that the current Codex definition for TFA does allow some flexibility within its interpretation. For example, Canada, the USA, China, South Korea, the Mercosur member countries including Argentina, Brazil, Paraguay, Uruguay and Venezuela, and some European countries such as Denmark, Iceland, Switzerland and Austria have implemented food-labelling regulations based on the current Codex TFA definition; however, variations among these countries exist in the method and type of regulations implemented. The main difference has been whether or not to apply mandates to unprocessed natural foods (e.g. whole-fat dairy products) that do not undergo industrial partial hydrogenation processes. Interestingly, the FSANZ has adopted the definition of TFA as all fatty acids containing trans double bond(s) with no exclusion of CLA at all(18).
Section summary
The purpose of the present review is to help clarify some of the major issues surrounding the implications of the Codex TFA definition. In particular, we wish to highlight how supplemental CLA is different from those derived from ruminant fat, and whether or not scientific advances continue to support the exclusion of CLA from the Codex TFA definition. Further, we raise the point that emerging data suggest rTFA differ from iTFA and how this may have an impact on the current interpretations of the Codex TFA definition.
Differences between supplemental conjugated linoleic acid and those derived from ruminant fat
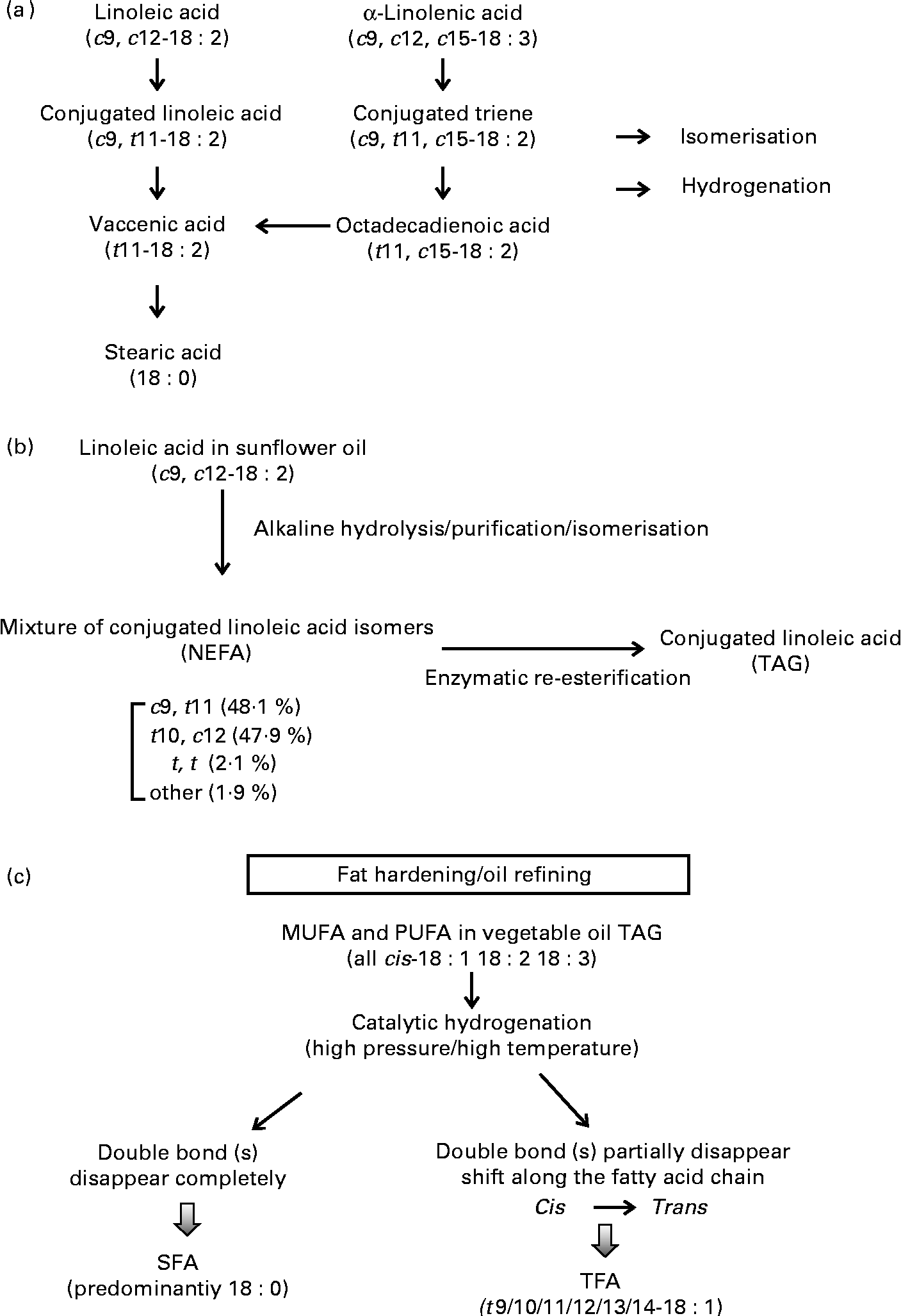
CLA has a similar chemical structure to linoleic acid (cis-9, cis-12-18 : 2), except that the conjugated double bonds are predominantly in positions 7 and 9, 8 and 10, 9 and 11, 10 and 12 or 11 and 13 in either the cis or trans configuration. The family of CLA can include up to twenty-eight possible different isomers, with two of these (i.e. cis-9, trans-11-CLA and trans-10, cis-12-CLA) known to possess bioactivity. Cis-9, trans-11-CLA is the most predominant isomer, present naturally as esterified fatty acids in the TAG of ruminant fat and dairy products. It is synthesised via biohydrogenation of linoleic/linolenic acid by ruminant bacteria and in vivo conversion from trans-11-vaccenic acid (VA) in the liver and adipose tissue of ruminant animals(Reference Palmquist, Lock and Shingfield19) (Fig. 1). In addition to its presence in ruminant-derived products, CLA is also available commercially in an enriched supplemental form (usually with a formulation of 80 % of the two CLA isomers cis-9, trans-11-CLA and trans-10, cis-12-CLA at a 1:1 ratio) and is typically produced from safflower oil rich in linoleic acid. A common method to produce supplemental CLA is to saponify food-grade safflower oil TAG to NEFA, further isomerised under conditions of high pH and temperature and then inter-esterified with glycerol to re-form TAG(Reference McCrorie, Keaveney and Wallace20) (Fig. 1). Some manufacturers also provide supplemental CLA in the free acid form. The finished CLA product typically contains a minimum of 78 % of total CLA isomers and at least 74 % of either a common 50:50 or a less common 80:20 mixture of cis-9, trans-11-CLA and trans-10, cis-12-CLA. In some countries (but not all), supplemental CLA has been accepted as generally safe at 1·5 g/serving up to 3 g/d for 2 years by the US Food and Drug Administration (GRN000232)(13) and 3–5 g up to 6 months by Health Canada(21). Although not accepted by the FSANZ, supplemental CLA has also been approved as a novel food ingredient by the European Food Safety Authority(12, 14, Reference Tetens22) at a dose of 3–3·5 g/d for up to 6 months in the general population, except in subjects diagnosed with type 2 diabetes.

Fig. 1 Schematics of dietary trans-fatty acids (TFA) from (a) natural ruminant biohydrogenation, (b) synthetic supplements and (c) industrial partial hydrogenation of vegetable oils.
The differences between supplemental and ruminant sources of CLA can be loosely categorised into the following: (1) isomer distribution; (2) consumption level; (3) regio-specific distribution in TAG molecules; (4) bioavailability. Supplemental CLA contain various isomers, with two being the most abundant (i.e. cis-9, trans-11-CLA and trans-10, cis-12-CLA), and the average recommended daily dose is 1100 mg for each of these two isomers (3 g of total CLA-rich oil). In contrast, CLA that is present naturally in ruminant-derived foods, such as beef, lamb and dairy products, differs greatly in the proportion of isomers compared with that of supplemental CLA, with the cis-9, trans-11-CLA isomer (also known as rumenic acid) being predominant (70–90 %) and only a trace amount as trans-10, cis-12-CLA(Reference Lock and Bauman23). The amount of cis-9, trans-11-CLA in ruminant sources (e.g. 2 % fat milk, butter, beef) can range from 5 mg/g fat with a standard feeding regimen to as high as 47 mg/g fat in enriched products(Reference Lock and Bauman23). The average dietary intake of ruminant CLA from natural food sources is approximately 100–180 mg/d in the UK and North America(Reference McCrorie, Keaveney and Wallace20), and may be 2–3-fold higher in certain European countries such as Germany, Denmark and The Netherlands (depending on population dietary patterns, geographical locations, forage conditions and other factors)(Reference Parodi24, Reference Fritsche and Steinhart25). The highest level reported (1000 mg/d) was observed in a Hare Krishna community in Australia due to a high consumption of ghee and butter(Reference Parodi24). It is also important to note that isomers from supplemental CLA can be present as either free acids or inter-esterified TAG at various sn positions based on synthetic conditions and substrate ratios(Reference Maurelli, Blasi and Cossignani26). In contrast, ruminant-derived CLA is incorporated considerably into the sn-1 position in phospholipids and over 50 % in the sn-3 position in milk TAG(Reference Chardigny, Masson and Sergiel27, Reference Valeille and Martin28). The positional distribution of CLA in ruminant muscle or adipose tissue can differ, with more incorporation into the sn-2 position of the TAG(Reference Paterson, Weselake and Mir29).
Variations in the bioavailability of CLA from supplemental and ruminant sources have been attributed to their presence as a free or esterified acid, the sn position of TAG as well as the characteristics of the food matrix they are consumed with. A number of studies have compared intestinal absorption of supplemental CLA isomers in different forms (i.e. NEFA, TAG or fatty acid ethyl esters) in rodents and human subjects. It has been reported that CLA is better absorbed as a TAG than a NEFA (which also tends to be more susceptible to oxidation)(Reference Yurawecz, Hood and Mossoba30–Reference Martin, Sebedio and Caselli32). Moreover, fatty acids incorporated into the sn-2 position of TAG tend to be more absorbed than either the sn-1 or -3 position(Reference Bracco33); but opposing results have also been reported for ruminant CLA, which has been found to be more bioavailable when in the external position (sn-1/3) than in the internal sn-2 position(Reference Chardigny, Masson and Sergiel27). Gervais et al. (Reference Gervais, Gagnon and Kheadr34) further reported that cis-9, trans-11-CLA was highly bioavailable from milk and the specific regiodistribution did not affect its intestinal digestibility.
Section summary
Differences exist between supplemental and ruminant sources of CLA including: isomer distribution, consumption level, regio-specific distribution in TAG and possibly bioavailability. Supplemental CLA contains two abundant isomers (i.e. cis-9, trans-11-CLA and trans-10, cis-12-CLA), whereas in ruminant-derived foods (such as beef, lamb and dairy products), the predominant isomer is cis-9, trans-11-CLA and only a trace amount as trans-10, cis-12-CLA(Reference Lock and Bauman23). The differences between these two forms/sources of CLA suggest that they should not be considered equal with respect to health regulations and/or nutritional guidelines.
Does the current literature (clinical and preclinical studies) suggest whether conjugated linoleic acid should be excluded from the Codex trans-fatty acid definition?
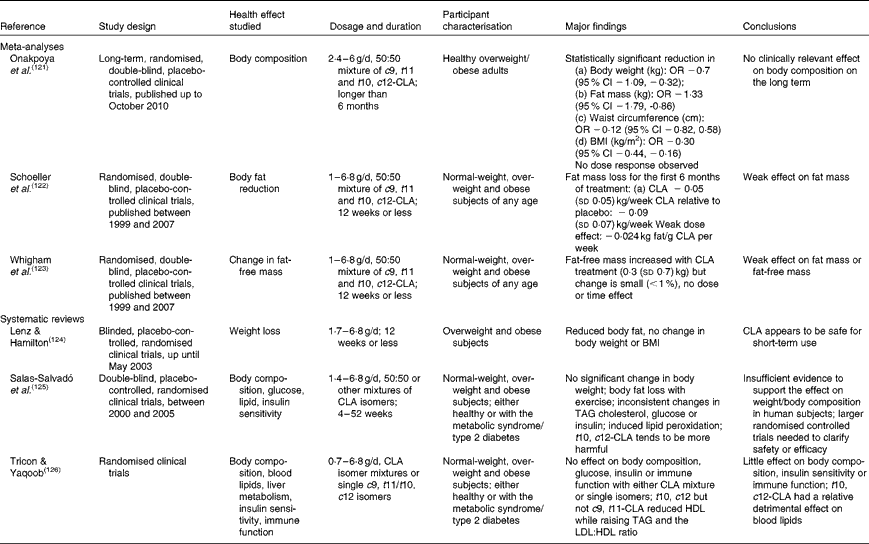
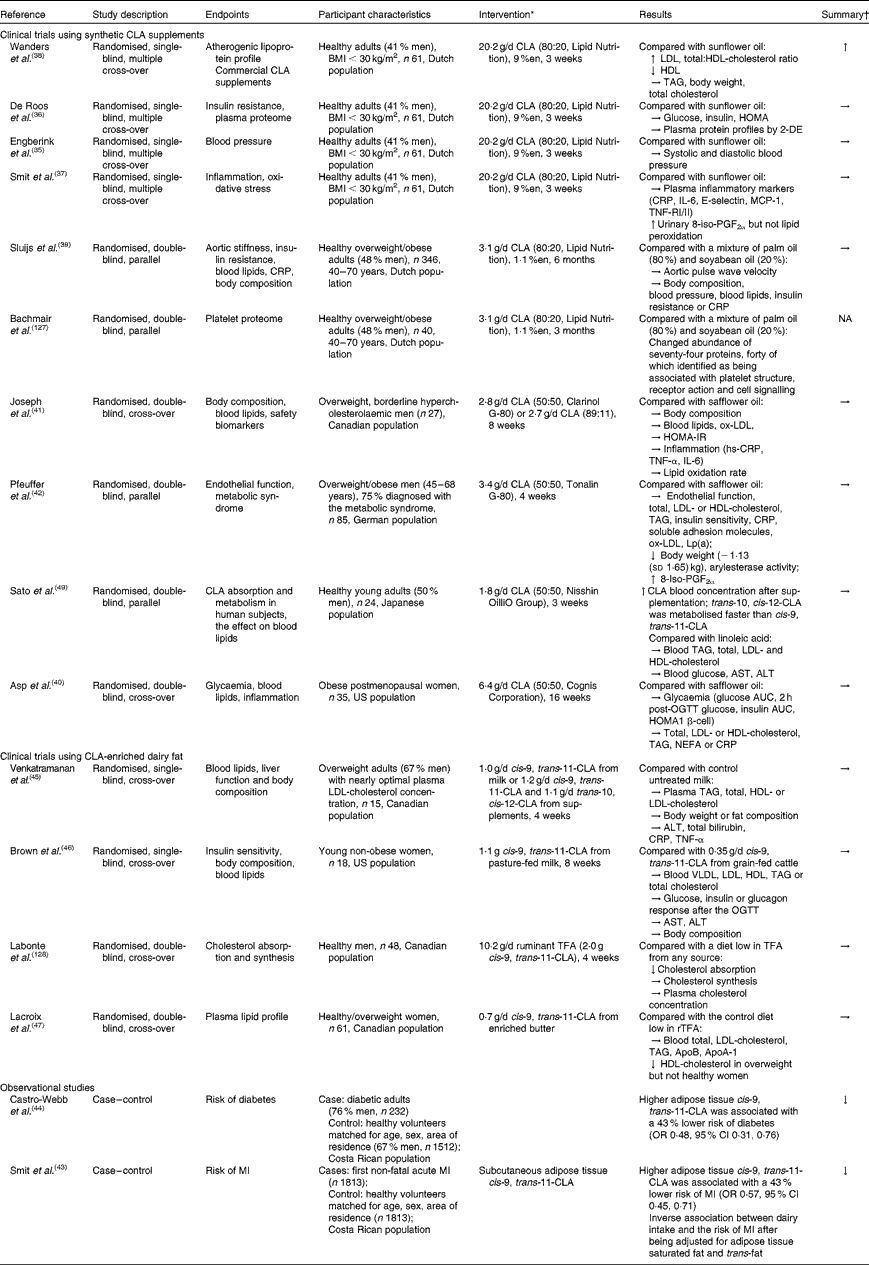
Independent reviews published before April 2010 to elucidate the effect of CLA in human subjects, with a primary focus on body-weight/fat reduction, are summarised in Table 1. A number of government regulatory bodies such as the FSANZ and the European Food Safety Authority have also generated reports on the safety of supplemental CLA as a potential ingredient for novel foods(14, Reference Tetens22). Despite different recommendations provided by these government reports, they are consistent in that CLA supplementation at a daily dose of less than 7 g showed little effect on clinically meaningful reduction in body weight or fat mass. In order to gather the most recent literature on CLA (with a specific focus for the definition of TFA), we have reviewed research published from April 2010 to November 2012 that have advanced this field (by searching the PubMed database using ‘conjugated linoleic acid’ and ‘CLA’). Only human studies using CLA as the primary investigating agent and with a focus of obesity and CVD-related endpoints were included in the following discussion (fourteen randomised clinical trials and two retrospective case–control studies; Table 2).
Table 1 Summary of meta-analyses and systematic reviews on the health effect of conjugated linoleic acid (CLA) in human subjects

Table 2 Summary of observational and intervention studies on the health effect of conjugated linoleic acid (CLA) in human subjects

%en, Percentage of energy; HOMA, homeostatic model assessment; 2-DE, two-dimensional electrophoresis; CRP, C-reactive protein; MCP-1, monocyte chemoattractant protein-1; TNF-R, TNF receptor; NA, not available; ox-LDL, oxidised LDL; HOMA-IR, HOMA of insulin resistance; hs-CRP, high-sensitivity CRP; Lp (a), lipoprotein (a); AST, aspartate aminotransferase; ALT, alanine aminotransferase; OGTT, oral glucose tolerance test; TFA, trans-fatty acids; rTFA, ruminant-derived TFA; MI, myocardial infarction.
* Doses refer to total CLA isomers as unesterified fatty acids; 50:50, 80:20 or 89:11 indicates the ratio of cis-9, trans-11-CLA:trans-10, cis-12-CLA in CLA supplements.
† Effect on the endpoints: ↑ increased; ↓ decreased; → neutral effect.
Supplemental conjugated linoleic acid and human health
The majority of recent clinical intervention studies have focused on the effect of supplemental CLA on cardiovascular risk parameters. Of these, four publications were generated from an intervention trial conducted in a group of healthy Dutch adults, each focusing on a different risk factor for CVD. Collectively, the results demonstrated that supplemental CLA (cis-9, trans-11:trans-10, cis-12-CLA, 80:20), relative to sunflower oil high in oleic acid, had no effect on blood pressure(Reference Engberink, Geleijnse and Wanders35), insulin sensitivity(Reference de Roos, Wanders and Wood36), plasma proteome(Reference de Roos, Wanders and Wood36), inflammatory markers or oxidative stress(Reference Smit, Katan and Wanders37) at a dose of 20·2 g/d for 3 weeks. Negative effects on lipoprotein profiles were observed in the same study, which include increased total cholesterol, LDL-cholesterol (LDL-C) and total:HDL-cholesterol (HDL-C) ratio compared with sunflower oil(Reference de Roos, Wanders and Wood36, Reference Wanders, Brouwer and Siebelink38). These adverse effects may probably be attributed to the high dosage (20·2 g/d, equivalent to about 9 % daily energy), since another Dutch study showed no such adverse outcomes using the same CLA preparation for a 7-fold longer duration (6 months) but at a lower dose (3·1 g/d, 1·1 % daily energy)(Reference Sluijs, Plantinga and de Roos39). Interestingly, a neutral effect was reported for supplemental CLA with a different isomer profile (cis-9, trans-11:trans-10, cis-12-CLA, 50:50) on body composition, blood lipid profile, endothelial function and inflammatory markers with effective doses varying from 1·8 to 6·4 g/d when compared with safflower oil(Reference Asp, Collene and Norris40–Reference Pfeuffer, Fielitz and Laue42).
Ruminant conjugated linoleic acid and human health
In two retrospective case–control studies, it was suggested that the adipose enrichment of cis-9, trans-11-CLA appeared to be protective against the future risk of non-fatal acute myocardial infarction and diabetes(Reference Smit, Baylin and Campos43, Reference Castro-Webb, Ruiz-Narvaez and Campos44). In a number of clinical intervention studies, cis-9, trans-11-CLA-enriched dairy fat at doses between 0·7 and 1·0 g/d did not appear to affect serum lipid or lipoprotein profile in normolipidaemic, yet overweight human subjects when consumed in moderation(Reference Venkatramanan, Joseph and Chouinard45–Reference Lacroix, Charest and Cyr47). Venkatramanan et al. (Reference Venkatramanan, Joseph and Chouinard45) compared the effect of milk naturally or synthetically enriched with cis-9, trans-11-CLA (1·1 g/d) on blood lipid indices, liver function and body composition in overweight human subjects. In this 8-week intervention study, conventional milk (0·2 g/d cis-9, trans-11-CLA) was used as the control and no significant changes were observed in both CLA-supplemented groups on all parameters measured. Similarly, neutral effects of ruminant CLA (from pasture-fed beef) on blood lipids and body composition were observed in healthy women in a US intervention study at the same dose and duration (cis-9, trans-11-CLA: 1·17 g/d for 8 weeks) relative to grain-fed ground beef(Reference Brown, Trenkle and Beitz46). In a group of healthy Canadian women, a cis-9, trans-11-CLA of 0·7 g/d for 4 weeks from rTFA-enriched butter showed the neutral effect on LDL relative to regular butter containing one-third of the rTFA content in enriched butter. However, we note that only one-quarter of the dose and half the duration were used in this Canadian study compared with the two clinical trials discussed earlier(Reference Lacroix, Charest and Cyr47). Further, the baseline characteristics of participants involved in the clinical trials indicate that fasting blood TAG, total cholesterol and LDL-C were well within the desirable or near optimal range according to the International Diabetes Federation and National Cholesterol Education Panel – Adult Treatment Panel (NCEP-ATP) III guidelines. The observed lack of the efficacy of cis-9, trans-11-CLA may possibly be due to: the relatively low consumption level of this isomer from food; the putative beneficial effects from control fats (e.g. sunflower oil high in oleic acid) on the same parameters measured; the lack of predisposed metabolic disorders in the studied population. We also acknowledge that the enrichment of cis-9, trans-11-CLA in dairy fat is accompanied by changes in other potentially bioactive fatty acids (e.g. trans-11-VA); potential healthy implications associated with such products could not be ascribed solely to cis-9, trans-11-CLA (discussed below).
Isomer-specific effect of conjugated linoleic acid from preclinical studies
One of the major differences between supplemental and ruminant CLA is the isomer composition. In order to delineate differential health effects associated with specific CLA isomers, publications included in the following discussion focused exclusively on individual isomers (i.e. cis-9, trans-11-CLA and trans-10, cis-12-CLA) rather than mixtures of CLA isomers. The studies currently available are predominantly from preclinical models rather than from human subjects. Notably, the general dose used in cited animal studies was 0·5 % (w/w) for each isomer (equivalent to approximately 1 % of daily energy), which appears to be much higher than the common doses used in human clinical trials (e.g. 3·1 g/d of 50:50 isomer mixture, 0·5 % daily energy for each isomer based on a 10 460 kJ (2500 kcal) diet). Similarly, the in vitro studies cited below generally used supraphysiological doses between 50 and 200 μmol/l, which are difficult to achieve even with supplementation(Reference Zlatanos, Laskaridis and Sagredos48, Reference Sato, Shinohara and Honma49). Therefore, caution should be applied when examining these preclinical data so as to avoid over-interpretation.
Anti-obesity effects
The potent effect of trans-10, cis-12-CLA present in supplemental CLA has been associated with reduced lipid content, the size and number of adipocytes in rats, mice and human subjects, as discussed in Declercq et al. (Reference Declercq, Taylor and Zahradka50) and Park et al. (Reference Park, Terk and Park51), but not in hamsters(Reference Lasa, Simon and Churruca52). The increased mobilisation of fatty acids from adipose tissue was found to be commonly associated with hepatic hypertrophy and steatosis, insulin resistance as well as increased inflammation and decreased de novo adipocyte lipogenesis(Reference Obsen, Faergeman and Chung53), without affecting adipose TAG lipase activity or fatty acid synthesis in mature adipocytes(Reference Lasa, Miranda and Churruca54). These changes appear to be mediated by a select expression pattern of key metabolic regulators including: increased proliferative signals in the liver(Reference Ashwell, Ceddia and House55, Reference Yu, Yu and Jiang56), suppressed myogenic differentiation and GLUT4 expression in the muscle(Reference Hommelberg, Plat and Remels57) as well as activated AMP-activated protein kinase and c-Jun N-terminal kinase signalling pathways in adipocytes(Reference Jiang, Chen and Wang58, Reference Martinez, Kennedy and McIntosh59) upon supplementation of trans-10, cis-12-CLA. However, no such effects were reported for the ruminant isomer cis-9, trans-11-CLA(Reference Zhai, Liu and Li60). Interestingly, trans-10, cis-12-CLA appeared to have an inconsistent effect on the content of lipid in the liver and systemic inflammation in fa/fa Zucker rats. Although one study suggested that trans-10, cis-12-CLA appeared to be beneficial(Reference Stringer, Zahradka and Declercq61), another two studies suggested adverse implications on liver morphology and function(Reference Declercq, Taylor and Zahradka50, Reference DeClercq, Zahradka and Taylor62).
Anti-cardiovascular effects
The cis-9, trans-11-CLA isomer, which is typically found in dairy products and beef, has recently been shown to reduce the expression of intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 on the surface of endothelial cells as well as to reduce macrophage adhesion to human umbilical vein endothelial cells in cell culture(Reference Stachowska, Siennicka and Baskiewcz-Halasa63). Cis-9, trans-11-CLA has also been found to reduce insulin resistance and associated inflammation in ob/ob mice, possibly by improving cellular endoplasmic reticulum stress and redox status(Reference Rungapamestry, McMonagle and Reynolds64). In addition, this ‘naturally occurring’ isomer has been shown to increase PPARγ activation and adipocyte differentiation by inhibiting extracellular signal-regulated protein kinases 1 and 2 phosphorylation, whereas trans-10, cis-12-CLA regulates macrophage metabolism via a different pathway (i.e. p38 phosphorylation) and mediates its apoptotic effect on mammary epithelial cells(Reference Hsu, Meng and Ou65, Reference Stachowska, Kijowski and Dziedziejko66). On the other hand, certain bioactivities in attenuating CVD risk have been associated with the trans-10, cis-12-CLA isomer but not the cis-9, trans-11-CLA isomer. Declercq et al. (Reference Declercq, Taylor and Zahradka50, Reference DeClercq, Zahradka and Taylor62, Reference Declercq, Taylor and Wigle67) published a series of studies using the fa/fa Zucker rat model (that have established obesity and hypertension). The authors have reported that purified trans-10, cis-12-CLA (but not cis-9, trans-11-CLA) effectively reduced systolic blood pressure by 17 mmHg at a dose of 0·4 % (w/w) for 8 weeks(Reference Declercq, Taylor and Wigle67). Changes in adiponectin levels further induced increased phosphorylated endothelial NO synthase in adipose tissue and the aorta(Reference Declercq, Taylor and Wigle67). Similar anti-hypertensive effects have also been observed in young Zucker rats in which trans-10, cis-12-CLA prevented the increase in systolic blood pressure(Reference Declercq, Taylor and Zahradka50). Trans-10, cis-12-CLA has also been associated with the potent immunoregulatory effect on inflammatory cells such as monocytes(Reference Kim, Kim and Kang68–Reference Perdomo, Santos and Badinga70) and polymorphonuclear neutrophilic leucocytes(Reference Paek, Kang and Kim71) in pigs and bovine animals.
Anti-carcinogenic effects
Preclinical studies that have used a synthetic mixture of CLA isomers during or after chemical carcinogen-induced tumorigenesis have implied anti-cancer efficacy in the mammary gland, colon and skin(Reference Kelley, Hubbard and Erickson72–Reference Heinze and Actis75). These differential effects of CLA on tumorigenesis have been primarily demonstrated in in vitro models including: colorectal cancer cells; MG63 osteosarcoma cells; MCF-7 breast cancer cells. In terms of isomer-specific effects, trans-10, cis-12-CLA (but not cis-9, trans-11-CLA) induced apoptosis via enhanced AMPK pathways independent of nutrient/energy depletion(Reference Hsu, Meng and Ou65, Reference Hsu and Ip76) in a p53-mutant rat mammary tumour cell model; however, in a different mammary cell line (MCF-10A), cis-9, trans-11-CLA has been shown to be a more effective anti-carcinogenic than trans-10, cis-12-CLA(Reference Rakib, Kim and Jang77). In the case of colorectal cancers, treatment of trans-10, cis-12-CLA was associated with suppressed proteasome activity and the accumulation of ubiquitinylated substrates in one of the most widely used human colorectal adenocarcinoma cell lines (CaCO2 cells)(Reference Palmieri, Bergamo and Luini78). However, none of these changes was observed when CaCO2 cells were treated with the cis-9, trans-11-CLA isomer at the same dose and duration. In a different colon cancer cell model, the enrichment of cis-9, trans-11-CLA in alpine milk lipids (2·7 % of fat as cis-9, trans-11-CLA) showed no additional growth-inhibitory effect in highly transformed HT-29 adenocarcinoma cells relative to conventional milk (0·3 % of total fat as cis-9, trans-11-CLA)(Reference Degen, Lochner and Keller79). An interesting study was conducted by Bassaganya-Riera & Hontecillas(Reference Bassaganya-Riera and Hontecillas80) that assessed the immunoregulatory mechanism of CLA in colorectal cancer, using either a commercial 50:50 CLA mixture or a probiotic mixture that synthesises predominantly cis-9, trans-11-CLA in the gut lumen of C57BL/6 wild-type mice(Reference Gorissen, Raes and Weckx81). This study showed that the probiotic mixture (with undetectable amounts of trans-10, cis-12-CLA) was more effective in decreasing inflammation and reducing disease activity in two colon carcinoma mouse models compared with the commercial CLA product(Reference Bassaganya-Riera and Hontecillas80). Nevertheless, data from human subjects on isomer-specific bioactivities remain to be limited and require further investigation.
Section summary
Literature published over the last 2–3 years remains consistent with earlier findings (i.e. before April 2010) that supplemental CLA regimens have shown little effectiveness to the reduction of body fat or CVD risk markers. This may be particularly relevant at higher doses or select population groups. In contrast, the cis-9, trans-11-CLA isomer from ruminants (in the form of conventional or moderately enriched dairy fat preparations) appears to be associated with neutral to beneficial health outcomes in humans. The consequence of these diverging observations underpins the increasing confusion for public health messaging and food labelling. Since supplemental CLA preparations are fundamentally different from CLA associated with food (and are usually consumed at substantially higher doses), we propose that concerns pertaining to CLA supplementation should be addressed separately from food-related issues and its usage be regulated independently as a nutraceutical or natural health product.
Do trans-fatty acids from ruminant and industrial sources have differential bioactivity?
iTFA isomers, often in the form of trans-18 : 1, originate from the refining process of vegetable oils or fat hardening, aiming at producing edible fat with a more pleasant colour, neutral flavour and odour(Reference Martin, Milinsk and Visentainer82) (Fig. 1). However, there are also various trans-18 : 2 fatty acids formed during the heating of vegetable oils in the refinery (e.g. during deodorisation)(Reference Kemeny, Recseg and Henon83). Industrial fats/oils contain appreciable amounts of non-conjugated trans-18 :2 fats, whereas on the contrary, ruminant-derived fats contain only traces(Reference Mozaffarian, Aro and Willett1, Reference Mozaffarian84). Trans-11–18 : 1 (VA) is the most predominant TFA isomer in ruminant fat when feeding a high proportion of forage, generally accounting for approximately 70 % of the total ruminant trans-fat(Reference Lock and Bauman23). Interestingly, in ruminants, rodents and humans, VA is also the major precursor for the endogenous synthesis of cis-9, trans-11-CLA(Reference Santora, Palmquist and Roehrig85, Reference Turpeinen, Mutanen and Aro86). In humans, approximately 19–30 % of dietary VA is converted to this natural CLA isomer(Reference Turpeinen, Mutanen and Aro86, Reference Bhattacharya, Banu and Rahman87). Although VA is also present in industrial fats, the contribution from these commercial sources to the total intake of VA is far below that attributable to seasonal variations of VA in ruminant fat(Reference Wolff, Combe and Destaillats88). While it is true that select TFA isomers are found in industrial partially hydrogenated vegetable oils as well as natural ruminant fat, the relative abundance of these individual fatty acid isomers differs significantly. In addition, we note that the majority of trans-18 : 1 isomers in industrial fats have their ethylenic bond between the Δ4 and Δ10 positions, whereas most trans-18 : 1 isomers in ruminant fats have their ethylenic bond at position Δ11 and beyond(Reference Wolff, Combe and Destaillats88). It is generally accepted that the TFA profiles of industrial and ruminant trans-fat are fundamentally different in their isomer distribution, stereochemistry, physical property as well as their abundance in food sources(Reference Gebauer, Chardigny and Jakobsen9).
Ruminant-derived trans-fatty acids v. industrial trans-fatty acids: epidemiological and clinical studies
Several epidemiological studies in Europe and the USA have released their latest findings on TFA intake and cardiovascular health outcomes. A few cross-sectional studies have reported a positive association between CVD incidence/major risk factors and trans-fat consumption primarily from processed vegetable oils(Reference Micha, King and Lemaitre89–Reference Varraso, Kabrhel and Goldhaber91). In the National Health and Nutrition Examination Survey (NHANES) cohort, plasma concentrations of all major TFA (both industrial and ruminant) and corresponding LDL-C have declined significantly following the successful implementation of trans-fat regulations(Reference Vesper, Kuiper and Mirel92). Unfortunately, a more detailed assessment of the association between LDL-C and individual TFA isomers using the NHANES cohort was not feasible due to limited information. However, a large-scale prospective cohort study in Norwegian counties conducted by Laake et al. (Reference Laake, Pedersen and Selmer93) has followed 70 000 people over 20 years, and the association of TFA from iTFA and rTFA with cardiovascular mortality assessed. The authors have reported that dietary TFA intake increased CVD risk irrespective of source, but that the association was not significant for ruminant trans-fat in either men or women after several major confounders were accounted for (e.g. dietary saturated fat and cholesterol)(Reference Laake, Pedersen and Selmer93). A recently published prospective cohort study in Denmark has further revealed a weak but significantly inverse association between rTFA consumption and weight change at lower intakes, which plateaued above a daily intake of 1·2 g(Reference Hansen, Berentzen and Halkjaer94). When specific iTFA isomers were studied, non-conjugated trans-18 : 2 have been shown to have a stronger positive relationship with CHD than for other trans-fats(Reference Micha, King and Lemaitre89, Reference Baylin, Kabagambe and Ascherio95, Reference Lemaitre, King and Mozaffarian96). On the contrary, cis-9, trans-11-CLA in adipose tissue that is linearly correlated with dairy intake(Reference Smit, Baylin and Campos43) was significantly lower in patients with diabetes (n 1512) relative to controls (n 232)(Reference Castro-Webb, Ruiz-Narvaez and Campos44). Only a few randomised controlled trials have ever been published using rTFA-enriched dairy fat, which collectively appear to have neutral health effects in normolipidaemic subjects (as discussed in the section ‘Ruminant conjugated linoleic acid and human health’). Unfortunately, no data have been published thus far using purified preparations of individual rTFA isomers in people with increased CVD risk.
Section summary
The findings from recent prospective cohort studies and randomised clinical trials are consistent with earlier systematic reviews(Reference Brouwer, Wanders and Katan2, Reference Bendsen, Christensen and Bartels10), showing that moderate consumption of rTFA at doses achievable by the diet alone has no adverse effect on CVD risk.
Ruminant-derived trans-fatty acids v. industrial trans-fatty acids: preclinical studies
The consumption of partially hydrogenated vegetable oil as the major source of iTFA in animal models has been shown to increase the atherogenic lipoprotein profile(Reference Kraft, Spiltoir and Salter97), blunt brain neurochemical synthesis(Reference Teixeira, Dias and Pase98) and induce hepatic steatosis, lipid peroxidation and hypertrophy(Reference Collison, Zaidi and Saleh99, Reference Dhibi, Brahmi and Mnari100). A high consumption of hydrogenated vegetable fat during pregnancy and lactation has also been shown to lead to hypothalamic inflammation and impaired satiety sensing, which promotes deleterious metabolic consequences such as obesity(Reference Pimentel, Lira and Rosa101). Impairment in brain function in iTFA-fed rats appears to be consistent with a cross-sectional clinical study that reported a decreased cerebral brain volume and worse cognitive function among those with higher plasma iTFA concentrations(Reference Bowman, Silbert and Howieson102). Interestingly, non-conjugated 18 : 2 iTFA have been associated with the induction of pro-inflammatory response, endothelial dysfunction(Reference Harvey, Arnold and Rasool103) and endothelial cell calcification(Reference Kummerow, Zhou and Mahfouz104), which in turn could accelerate the development of CVD.
A number of recent in vitro cell-culture studies have provided an updated perspective in support of the discretionary bioactivity on cellular metabolic pathways between major rTFA and iTFA isomers. Iwata et al. (Reference Iwata, Pham and Rizzo105) assessed two major iTFA (elaidic acid (EA, trans-9-18 : 1) and linoelaidic acid (trans-9, trans-12–18 : 2)) and the most abundant rTFA (i.e. trans-11–18 : 1, VA) regarding their individual effect on endothelial function. EA and linoelaidic acid were associated with the increased NF-κB activation and impairment of endothelial insulin signalling and NO production, consistent with previously reported endothelial dysfunction for industrial trans-fat in human subjects(Reference Harvey, Arnold and Rasool103, Reference Mozaffarian106). On the contrary, such adverse effects were not observed in cells treated with VA. In another in vitro study, treatment of EA (but not VA) was associated with impaired cholesterol efflux from mouse and human macrophages(Reference Fournier, Attia and Rousseau-Ralliard107). The authors have accredited the changes to reduced long-chain PUFA incorporation into membrane phospholipids, thus altered membrane fluidity in EA-treated macrophages(Reference Fournier, Attia and Rousseau-Ralliard107). The negative effect of EA on n-3 long-chain PUFA incorporation is consistent with a recent cross-sectional study assessing maternal trans-fat intake and corresponding fetal blood fatty acid composition(Reference Enke, Jaudszus and Schleussner108). The distinctive bioactivity on membrane PUFA incorporation between VA and major iTFA isomers and subsequent changes in cell signalling pathways may be explained by earlier studies that have demonstrated that EA (and to a lesser extent linoelaidic acid) are potent inhibitors of Δ5 desaturation (critical for the biosynthesis of n-3 and n-6 PUFA). No such effect was shown for VA(Reference Rosenthal and Doloresco109).
Bioactivity of ruminant trans-fatty acids–trans-11-vaccenic acid
There is consistent evidence that purified VA supplementation (6·7 % of total fat) substantially improves atherogenic lipid profiles (e.g. TAG, LDL-C, total cholesterol) and improves hepatic steatosis in animal models of dyslipidaemia and the metabolic syndrome(Reference Tyburczy, Major and Lock110–Reference Van Nieuwenhove, Cano and Perez-Chaia116). It has been further proposed that VA binds to and functionally activates PPARα and γ, both of which are common targets for lipid-lowering and anti-diabetic medications such as fenofibrates and thiazolidinedoines, respectively(Reference Wang, Jacome-Sosa and Ruth117). In vitro cell-culture studies have also confirmed that VA does not have the same bioactivity as those from partially hydrogenated vegetable oils, such as EA(Reference Iwata, Pham and Rizzo105, Reference Fournier, Attia and Rousseau-Ralliard107). Furthermore, treatment of purified VA at physiological doses (40 μm) has been shown in vitro to effectively attenuate the development cardiomyocyte hypertrophy by activating PPARα/γ-dependent pathways(Reference Wang, Jacome-Sosa and Ruth117). As discussed earlier in the present review, evidence from randomised clinical trials so far has indicated that CLA/VA-enriched dairy fat can elicit neutral effects on blood lipid variables (LDL-C, HDL-C, total:HDL-C ratio) relative to iTFA in healthy individuals(Reference Lacroix, Charest and Cyr47, Reference Chardigny, Destaillats and Malpuech-Brugere118, Reference Motard-Belanger, Charest and Grenier119). Most recently, VA/CLA-enriched dairy fat has been shown to exert a neutral impact on peripheral insulin sensitivity in overweight women, but not significantly different from industrial sources of trans-fat(Reference Tardy, Lambert-Porcheron and Malpuech-Brugere120).
Section summary
Recent clinical and preclinical data continue to demonstrate a positive correlation between the consumption of industrial trans-fats and CVD risk measures, whereas this is not the case with a moderate intake of TFA from ruminant sources.
Concluding remarks
As the intake of dietary iTFA gradually declines, the proportion of rTFA to total TFA consumption will subsequently increase, suggesting that a clear understanding of both these forms of TFA will be critical for accurate public health policy. The current Codex definition of TFA encompasses the mandate to reduce the dietary intake of deleterious iTFA, but does not necessarily reflect emerging evidence suggesting differential health implications between iTFA and rTFA. We conclude that health concerns associated with the use of supplemental CLA do not repudiate the exclusion of all forms of CLA from the Codex TFA definition, particularly when using the definition for food-related purposes. Given the emerging differential bioactivity of TFA from industrial v. ruminant sources, we advocate that regional nutrition guidelines/policies should focus on eliminating industrial forms of trans-fat from processed foods as opposed to all TFA per se.
Acknowledgements
S. D. P. received research grants from the Alberta Meat and Livestock Agency and the Dairy Farmers of Canada. He also received recognition for his research from the International Dairy Federation. S. D. P. holds a New Investigator Award from the Heart and Stroke Foundation of Canada. Both authors contributed equally to the present review. Neither author has a conflict of interest with the work described herein.