Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis, is characterised by chronic inflammation of the gastrointestinal tract and by periods of relapse and remission. IBD patients are also at a higher risk of colorectal cancer. The incidence rates of IBD are between three and fifteen cases per 100 000 persons in the Western world, but there is also an increasing trend in low-incidence regions( Reference Lakatos 1 , Reference Loftus 2 ).
A combination of genetic, environmental and immunological factors contributes to the pathogenesis of IBD. It seems that an abnormal immune response to the normal microflora of the gut and an imbalance between pro-inflammatory and anti-inflammatory factors underlie the clinical features. The excessive inflammatory response includes the expression of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-8 and adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)( Reference Viscido, Aratari and Maccioni 3 , Reference Bouma and Strober 4 ). Oxidative stress is also present due to the excessive presence of oxidised molecules and the gradual depletion of antioxidant mechanisms. As a consequence, lipid peroxidation and oxidative DNA damage are increased in IBD patients( Reference D'Odorico, Bortolan and Cardin 5 , Reference Beltrán, Nos and Dasí 6 ).
Medical management aims to induce and maintain remission with the use of drugs such as aminosalicylates (e.g. sulfasalazine), corticosteroids (e.g. hydrocortisone), immunomodulators (e.g. azathioprine), antibiotics (e.g. metronidazole) and biological agents acting against TNF-α (e.g. infliximab)( Reference Pithadia and Jain 7 ). These biological agents are effective both in the induction of remission and in the preservation/recovery of the intestine( Reference Feagan, Lémann and Befrits 8 ).
However, since the currently used therapies are not always successful and may cause important side effects, there is an increasing interest in the role of nutritional therapy in the management of IBD. In particular, defined enteral nutrition (EN) seems to be able to play a critical role in the induction and maintenance of remission through mucosal healing and decrease of pro-inflammatory cytokine levels( Reference Forbes, Goldesgeyme and Paulon 9 ). The optimal composition of a therapeutic EN formula and, especially, the role of fat type in the effectiveness of EN remain controversial. Regarding the amount of long-chain TAG (LCT), some studies have shown a negative effect from increased LCT content( Reference Bamba, Shimoyama and Sasaki 10 , Reference Middleton, Rucker and Kirby 11 ), while a meta-analysis has shown no difference between high- and low-LCT formulae( Reference Zachos, Tondeur and Griffiths 12 ). However, LCT induce a greater inflammatory response than medium-chain TAG (MCT)( Reference Andoh, Takaya and Araki 13 ), thus suggesting that MCT may be preferable, and in a rat colitis model, MCT supplementation has been shown to result in decreased inflammation in the intestinal mucosa( Reference Tsujikawa, Ohta and Nakamura 14 , Reference Tsujikawa, Ohta and Nakamura 15 ). In addition, n-6 and n-3 PUFA have different impacts on inflammatory mechanisms, and some experimental studies of colitis have shown the beneficial effects of n-3 PUFA on inflammation, colonic damage and oxidative stress( Reference Hassan, Ibrahim and Mbodji 16 , Reference Kono, Fujii and Ogiku 17 ). Prompted by these data and the debated role of fat type in the nutritional management of IBD, the aim of the present study was to assess the effects of two commercially available isoenergetic EN formulae with different fat composition (MCT and LCT; n-6:n-3 ratio) on a rat model of colitis that resembles human IBD.
Experimental methods
Materials
Trinitrobenzene sulphonic acid (TNBS), butylated hydroxytoluene, PBS and Bradford reagent were obtained from Sigma-Aldrich Company. Xylazine was obtained from Pfizer Company. Ketamine and pentobarbital (Dolethal®) were purchased from Vétoquinol Company. All the ELISA kits used for the determination of ICAM-1, IL-6, IL-8 and IL-10 levels were obtained from R&D Systems. The malondialdehyde reagent kit was obtained from OxisResearch. The glutathione S-transferase (GST) assay kit was purchased from IBL International GmbH. Emsogen® and Elemental 028 Extra® were purchased from Nutricia Limited.
Experimental animals and diets
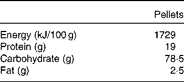
Adult male Wistar rats were obtained from the Greek Pasteur Institute and were kept in stainless steel cages at constant environmental conditions (22°C temperature and 50 % humidity) under a 12 h day–12 h night cycle. An acclimatisation period of 1 week preceded the experiment, during which all the rats were fed a standard pelleted diet (Vergerio Mangimi srl), the composition of which is given in Table 1. Access to food and water was free. The study was approved by the Animal Care Committee and was in agreement with the European Union Act and Greek Law 160, A-64, May 1991.
Table 1 Composition of the oral pelleted diet
A total of forty-five rats weighing 413·9 (sem 17·2) g were randomly assigned to five groups of nine rats as follows: group A – control, healthy, untreated rats; group B – TNBS-induced colitic rats; group C – TNBS-induced colitic rats fed Elemental 028 Extra®; group D – TNBS-induced colitic rats fed Emsogen®; group E – TNBS-induced colitic rats treated with infliximab.
Rats in groups A, B and E continued to be fed the baseline pelleted diet. Rats in groups C and D were fed elemental diets diluted in water (2:1) for 12 d before the induction of colitis and during the measurement of TNBS activity thereafter. The composition of the elemental diets is given in Table 2. Overall, the energy, protein, carbohydrate and fat content was virtually the same. In terms of fat composition, one elemental diet contained more LCT (65 %) and less MCT (35 %) and had a ratio of n-6:n-3 PUFA of 4:1, while another elemental diet was higher in MCT (83 %) than in LCT (17 %) and had a higher ratio of n-6:n-3 PUFA (46·5:1).
Table 2 Composition of the elemental diets
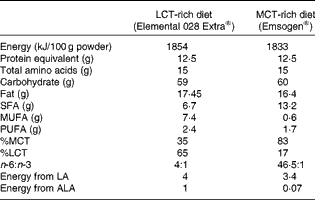
LCT, long-chain TAG; MCT, medium-chain TAG; LA, linoleic acid; ALA, α-linolenic acid.
Induction of trinitrobenzene sulphonic acid colitis
On day 0, the rats were weighed. All the rats, except those in group A, were placed in a cage with 8 % sevoflurane for 1 min to cause mild anaesthesia and muscle relaxation. Next, TNBS solution (100 mg/kg body weight (BW)) in 0·25 ml of 50 % ethanol was administered intracolonically through a venous-type catheter. The rats were kept in a vertical, head-down position for 2 min to prevent leakage. Rats in group E were injected subcutaneously with infliximab (5 mg/kg BW) 24 h after the administration of TNBS. On day 4, the BW of all the rats was recorded, and they were anaesthetised intramuscularly with ketamine–xylazine solution (80 and 16 mg/kg BW, respectively) in normal saline. Subsequently, after incision into the peritoneal cavity, 6 cm of the colon was removed from immediately above the rectum. Each specimen was separated into three parts and stored for the (1) assessment of histology, (2) evaluation of inflammation and (3) evaluation of oxidative stress. At the completion of the experimental period, the rats were killed by the administration of dolethal directly into the right ventricle of the heart (0·6 mg/kg BW).
Histological analysis
Colonic samples (2–5 cm) were fixed in 10 % buffered formalin. Representative sections were collected every 5 mm, deliberately including areas with any visually apparent damage. The sections were embedded after dehydration into paraffin blocks, and tissue sections (4–5 μm thick) were stained with haematoxylin and eosin. The samples were examined blindly for histological damage by two pathologists according to a previously described protocol( Reference Geboes, Riddell and Ost 18 ), and the following criteria were assessed: (0) architectural damage – 0·0 = no abnormality, 0·1 = mild abnormality, 0·2 = moderate diffuse or multifocal abnormalities, and 0·3 = severe diffuse or multifocal abnormalities; (1) chronic inflammatory infiltrate– 1·0 = no increase, 1·1 = mild but unequivocal increase, 1·2 = moderate increase, and 1·3 = marked increase; (2) eosinophils and neutrophils – 2·0 = no increase, 2·1 = mild but unequivocal increase, 2·2 = moderate increase, and 2·3 = marked increase; (3) neutrophils in epithelium – 3·0 = none, 3·1 = < 5 % crypts involved, 3·2 = < 50 % crypts involved, and 3·3 = >50 % crypts involved; (4) crypt destruction – 4·0 = none, 4·1 = probable local excess of neutrophils in part of the crypt, 4·2 = probable marked attenuation, and 4·3 = unequivocal crypt destruction; (5) erosion or ulceration – 5·0 = none, 5·1 = recovering epithelium+adjacent inflammation; 5·2 = probable erosion focally stripped, 5·3 = unequivocal erosion, and 5·4 = ulcer or granulation tissue.
Colonic tissue homogenisation and total protein quantification
The tissue samples were weighed and homogenised with PBS (1 ml/2 g tissue) and butylated hydroxytoluene (10 μl/ml homogenised tissue) with an Ultra-Turrax (IKA-Labortechnik) homogeniser. Butylated hydroxytoluene was added to prevent the self-oxidation of samples during homogenisation. Centrifugation was carried out (3000 rpm at 4°C) for 15 min to obtain the tissue extract, which was then kept at − 80°C until assay. The total protein content was quantified by the Bradford assay( Reference Bradford 19 ).
Evaluation of inflammation
The inflammatory state of the tissue homogenates was assessed through the measurement of ICAM-1, IL-6, IL-8 and IL-10 levels according to the instructions of the ELISA kit manufacturer (R&D Systems). Their concentrations are expressed as pg/mg of total protein.
Assessment of oxidative stress
Oxidative stress was evaluated by measuring malondialdehyde and GST levels, according to the manufacturer's instructions for their respective assays (OxisResearch and IBL International). Results for malondialdehyde are expressed as pmol/mg of total protein, while GST activity is expressed as nmol/min per ml.
Statistical analysis
The results are expressed as means with their standard errors. Statistical analysis was carried out by applying the statistical package: SPSS Statistics, version 17.0 (SPSS, Inc.). For comparisons of means, one-way ANOVA with Bonferroni's post hoc analysis was applied. Non-parametric data were analysed with the Kruskal–Wallis test. A P value < 0·05 was set as the level to determine significance.
Results
Macroscopic observations and body weight
Macroscopically, TNBS induced changes in stool consistency, with softer stools seen in group B compared with group A. No significant changes in food intake or in BW were observed between the groups as recorded on days − 12, 0 and 4.
Histological assessment
The histological evaluation of the colonic samples revealed a significant difference between the histological damage scores in groups A and B, confirming the induction of inflammation in the rat model used in the present study (P= 0·0001). The dietary treatment of rats in group C elicited a numerical increase in damage scores compared with that of rats in group B, but this was not statistically significant. No significant differences were observed between rats in group B and those in group D or E, but in these rats, the histological damage scores were numerically lower than those of the TNBS-alone rats in group B (Table 3). The results of the immunohistochemical analysis are shown in Fig. 1.
Table 3 Histological damage scores in healthy and colitic rats 4 d after the administration of trinitrobenzene sulphonic acid (TNBS) (Mean values with their standard errors)
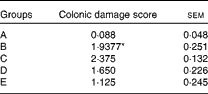
A, control, healthy, untreated rats; B, TNBS-induced colitic rats; C, TNBS-induced colitic rats fed Elemental 028 Extra®; D, TNBS-induced colitic rats fed Emsogen®; E, TNBS-induced colitic rats administered infliximab.
* Mean value was significantly different from that of group A (P= 0·0001).

Fig. 1 Results of the immunohistochemical analysis of sections prepared from colonic tissues (125 × original magnification). Pictures are representative of microscopic features in individual groups: (a) group A – healthy colonic mucosa; (b) group B – mucosal ulceration, fibrinopurulent exudate, and crypt destruction; (c) group C – chronic and active inflammation in the dermis and epithelium, ulceration, and crypts destruction; (d) group D – ulceration, crypt destruction, and presence of neutrophils and eosinophils and (e) group E – moderate chronic inflammation and presence of neutrophils. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Inflammatory markers
As expected, the inflammatory markers in group A were at low background levels. In group B, IL-6 (114 (sem 11) v. 65 (sem 6) pg/mg protein), IL-8 (62 (sem 4) v. 22 (sem 6) pg/mg protein) and ICAM-1 (10 104 (sem 412) v. 4483 (sem 74) pg/mg protein) levels were elevated, significantly so in the case of ICAM-1 (P= 0·0001), compared with those in group A (Fig. 2). The levels of the immunoregulatory cytokine IL-10 were significantly lower in group B than in group A (1·5 (sem 0·3) v. 6 (sem 0·8) pg/mg protein; P= 0·001).

Fig. 2 Inflammatory markers in colonic tissue homogenates. (a) Levels of IL-6, (b) levels of IL-8, (c) levels of intercellular adhesion molecule-1 (ICAM-1) and (d) levels of IL-10. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from those of group A (P< 0·05). † Mean values were significantly different from those of group B (P< 0·05). A, control, healthy, untreated rats; B, TNBS-induced colitic rats; C, TNBS-induced colitic rats fed Elemental 028 Extra®; D, TNBS-induced colitic rats fed Emsogen®; E, TNBS-induced colitic rats administered infliximab.
In group C, ICAM-1 levels were significantly lower than those in group B (4211 (sem 167) v. 10 104 (sem 412) pg/mg protein; P= 0·0001). In group D, IL-6, IL-8 and ICAM-1 (72 (sem 2) v. 114 (sem 11) pg/mg protein, P= 0·034; 5 (sem 1) v. 62 (sem 4) pg/mg protein, P= 0·0001; 3306 (sem 170) v. 10 104 (sem 412) pg/mg protein, P= 0·0001, respectively) levels were significantly lower than those in group B. Rats in group E had significantly lower IL-8 and ICAM-1 levels (26 (sem 3) v. 62 (sem 4) pg/mg protein, P= 0·032; 4356 (sem 122) v. 10 104 (sem 412) pg/mg protein, P= 0·0001) than those in group B and had significantly higher IL-10 levels (6 (sem 0·6) v. 1·5 (sem 0·3) pg/mg protein; P= 0·0001).
Markers of oxidative stress
Malondialdehyde levels in groups C, D and E were numerically lower than those in group B, but no significant differences were observed (Fig. 3). Rats in group B rats exhibited much higher GST activity than those in group A (74 (sem 1·7) v. 1·7 (sem 0·2) nmol/min per ml; P= 0·0001). GST activity was significantly less in groups C, D and E than in group B (26·2 (sem 4·5) v. 74 (sem 1·7) nmol/min per ml, P= 0·0001; 15·8 (sem 0·6) v. 74 (sem 1·7) nmol/min per ml, P= 0·0001; 19·2 (sem 0·6) v. 74 (sem 1·7) nmol/min per ml, P= 0·0001; 25·6 (sem 1) v. 74 (sem 1·7) nmol/min per ml, P= 0·0001, respectively).

Fig. 3 Oxidative stress markers in colonic tissue homogenates. (a) Levels of glutathione S-transferase (GST) and (b) levels of malondialdehyde (MDA). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from those of group A (P< 0·05). † Mean values were significantly different from those of group B (P< 0·05). A, control, healthy, untreated rats; B, TNBS-induced colitic rats; C, TNBS-induced colitic rats fed Elemental 028 Extra®; D, TNBS-induced colitic rats fed Emsogen®; E, TNBS-induced colitic rats administered infliximab.
Discussion
As the standard medical management of IBD includes numerous side effects and is not always effective, there is an increasing interest in the possible anti-inflammatory effects of natural products such as dietary constituents( Reference Kaliora, Stathopoulou and Triantafillidis 20 , Reference Kwon, Hwang and Lee 21 ) and EN. The optimal fat content of EN formulae remains a controversial issue, not least since the type of fat may interact differentially when there are polymorphisms in the cytokines regulating disease activity( Reference Guerreiro, Ferreira and Tavares 22 ). Thus, the objective of the present study was to evaluate the efficacy of two EN formulae with differing fat content in a rat model of colitis.
The results show that a formula rich in MCT ameliorates TNBS-induced colitis in rats. Treatment with the MCT-rich diet yielded significantly less increase in IL-6, IL-8 and ICAM-1 levels and GST activity, an effect comparable to that of infliximab. The LCT-rich diet did not show such an activity and suppressed only ICAM-1 levels and GST activity. Although the histological data were not so compelling, the present results indicate that an EN formula rich in MCT may be more beneficial than other formulae in IBD.
The TNBS-colitis model( Reference Morris, Beck and Herridge 23 ) is self-evidently not identical to human IBD, but its similarity and robustness have led to its widespread use in the investigation of IBD pathogenesis and in the assessment of new potential therapeutic agents. In this model, ethanol increases intestinal permeability so that TNBS reaches the subepithelial space. The resulting severe inflammatory response disrupts T-cell tolerance to mucosal antigens and alters T-cell localisation in the lamina propria, intraepithelial space and lymphoreticular tissues. TNBS colitis is characterised by macrophage, polymorphonuclear leucocyte and lymphocyte infiltration and ulceration( Reference Morris, Beck and Herridge 23 ). There is CD-like intestinal inflammation with the secretion of IL-1, IL-6, IL-12, IL-18, TNF-α and interferon-γ by activated T helper 1 cells and of IL-4, IL-5 and IL-13 by T helper 2 cells( Reference Velde, Verstege and Hommes 24 ). The significant differences in inflammatory markers and the histological damage scores in the TNBS-induced colitis group compared with those in the control group confirm the effective induction of TNBS colitis in the present study.
The levels of IL-6, which promotes apoptosis resistance and T-cell proliferation in the lamina propria, are significantly increased in the serum of CD patients( Reference Mudter and Neurath 25 ). A pilot trial of a humanised monoclonal antibody to the IL-6 receptor in active CD patients has demonstrated an increase in the response rate in the treated group compared with that in the placebo group( Reference Ito, Takazoe and Fukuda 26 ). In the present study, the MCT-rich diet, but not the LCT-rich diet (which has much less MCT), induced a significant relative decrease in IL-6 levels, indicating that the MCT content may be the key factor. Notably, this observation exceeds the effect observed with infliximab. The levels of IL-8, a chemokine responsible for the accumulation and stimulation of polymorphonuclear neutrophils at sites of inflammation( Reference Ina, Kusugami and Yamaguchi 27 ) and which is overexpressed in patients with active CD( Reference MacDermott 28 ), were also suppressed by the MCT-rich diet.
The levels of ICAM-1, a molecule promoting the adhesion between leucocytes and endothelial cells and present in higher concentrations in IBD( Reference Danese, de la Motte and Sturm 29 , Reference Nielsen, Langholz and Hendel 30 ), were suppressed by all the anti-inflammatory strategies used in the present study, but dietary therapy seemed less able to yield an IL-10 immunoregulatory response.
Oxidative stress has been suggested as a possible aetiological factor in IBD pathogenesis( Reference Kruidenier and Verspaget 31 – Reference Girgin, Karaoglu and Erkuş 35 ). We found no direct evidence to support this from our model. However, induction of GST activity was observed and its suppression by each of the anti-inflammatory strategies was demonstrated.
Although the mechanisms underlying the efficacy of elemental formulae include the exclusion of food antigens and change of the colonic bacterial environment( Reference Shah 36 ), the contrasting effects of two diets differing mainly in their lipid composition suggest that the nature of the dietary fat is also important, first proposed by Fernández-Bañares et al. ( Reference Fernández-Bañares, Cabré and González-Huix 37 ). The MCT-rich diet exerted stronger anti-inflammatory effects than the LCT-rich diet, which may be explained by the leucocyte immunomodulatory properties of MCT( Reference Waitzberg, Bellinati-Pires and Yamaguchi 38 , Reference Wanten, Geijtenbeek and Raymakers 39 ). MCT is known to regulate mucosal damage and inflammation( Reference Tsujikawa, Ohta and Nakamura 14 , Reference Tsujikawa, Ohta and Nakamura 15 , Reference Kono, Fujii and Ogiku 17 , Reference Ohta, Tsujikawa and Nakamura 40 ). Two independent surveys have shown a remarkable inverse correlation between remission rates in active CD and the LCT content of elemental feeds( Reference Middleton, Rucker and Kirby 11 , Reference Evans and Rhodes 41 ). Animal studies have shown that LCT absorption is associated with enhanced migration of lymphocytes, up-regulation of adhesion molecule levels and stimulation of lymphocyte function( Reference Miura, Tsuzuki and Hokari 42 ). A meta-analysis supports the absence of a difference in inflammatory response between groups with low and high LCT content( Reference Zachos, Tondeur and Griffiths 12 ). The trial carried out by Leiper et al. ( Reference Leiper, Woolner and Mullan 43 ) showed no difference in the effect of low-LCT and high-LCT whole-protein feeds in active CD, leading to the hypothesis that all previously reported correlations between the LCT content of the diet and response rates in active CD are unlikely to be due to LCT itself and may be due to some other component of high-LCT feeds. It is highly probable that the effects reported herein are a consequence of the MCT content of the diet.
It is acknowledged that our model differs from the clinical context in that the experimental diets were administered before, as well as after, the induction of IBD. However, this is an established methodology when aiming to evaluate the effectiveness of diet in the TNBS-colitis model, also given that the effect of TNBS diminishes rapidly within 4–5 d of administration. An interpretation that MCT is acting primarily in a prophylactic capacity receives support from another rat study (of MCT and n-3 PUFA)( Reference Kono, Fujii and Ogiku 17 ), but unguarded extrapolation should obviously be avoided.
The MCT-rich diet also has a higher n-6:n-3 PUFA ratio. Excess n-6 lipids have traditionally been considered to exacerbate inflammation, and high levels have been reported to be positively correlated with the CD activity index( Reference Kuroki, Iida and Matsumoto 44 ). Nevertheless, there are several studies in rat models of colitis that fail to demonstrate significant negative effects of a high n-6:n-3 PUFA ratio( Reference Ramakers, Mensink and Verstege 45 – Reference Bertevello, De Nardi and Torrinhas 47 ). The content of EPA and DHA is also important when considering the effectiveness of PUFA-rich diets in the inflammatory response. However, so far, the majority of studies investigating the anti-inflammatory effects of n-3 PUFA have utilised fish oils, with a heterogeneous mixture of EPA and DHA, and only a small number of studies have shown that pure DHA and/or EPA can reduce inflammation in vitro ( Reference Ibrahim, Mbodji and Hassan 48 ) or in experimental animals( Reference Cho, Chi and Chun 49 ). The precise content of DHA and EPA in the feeds that we used has not been established with confidence (it is not present in the product labelling), and any hypothesis on their effects would be speculative.
However, it would be oversimplistic to attribute benefits only to MCT, as alteration of the SCFA and MUFA may also be important( Reference Hou, Abraham and El-Serag 50 , Reference Sakamoto, Kono and Wakai 51 ). Indeed, our own, recent animal data( Reference Bertevello, De Nardi and Torrinhas 47 ) have shown that intravenous infusions of a MUFA-rich lipid emulsion increase IL-6 levels and induce more histological damage than MCT- and n-6 PUFA-rich emulsions. The MCT emulsion had the most desirable effects. Additionally, a major clinical trial( Reference Gassull, Fernandez-Banares and Cabre 52 ) in patients with active CD has demonstrated significantly lower remission rates after the consumption of an enteral formula rich in MUFA than after that of a formula rich in linoleic acid (15 % of total energy in the LCT-rich diet compared with 1 % in the MCT-rich diet).
The apparent beneficial impact of a diet rich in MCT, low in MUFA and relatively low in PUFA (either n-3 or n-6) in TNBS colitis supports the premise that modified fat EN may be a more effective choice for IBD patients than a standard feed. However, current guidance on therapeutic nutrition considers only Crohn's disease and, even there, places almost all of the emphasis on the treatment of small bowel disease. The intrinsic properties of the TNBS model suggest that dietary evaluation in Crohn's colitis and indeed in ulcerative colitis should also be revisited.
Acknowledgements
The authors thank the Experimental Research Unit of ELPEN-Pharmaceuticals for animal breeding and husbandry.
A. F. acknowledges support from the Biomedical Research Centre funding of UCL and its partner hospitals from the UK National Institute for Health Research. Biomedical Research Centre funding of UCL and partner hospitals from the UK National Institute for Health Research had no role in the design, analysis or writing of this article.
The authors' contributions are as follows: E. P. conducted the experimental and statistical analyses and wrote the first draft of the manuscript; A. C. K. and A. F. designed the protocol and edited the manuscript; A. G. and A. P. conducted the experimental analysis and contributed to the interpretation of the results. All the authors contributed substantially to the present study.
None of the authors has any conflicts of interest to declare.








