Introduction
Anthropogenic disturbances in their most diverse forms can directly and/or indirectly impact the survival of species (Caro Reference Caro2007; Wilson et al. Reference Wilson, Ridlon, Gaynor, Gaines, Stier and Halpern2020). For example, animals that inhabit anthropised environments, such as urban centres, may exhibit more aggressive behaviours at the expense of maternal care toward their young (Moroni et al. Reference Moroni, Crivelaro, Soares and Guillermo‐Ferreira2017), and sometimes lactating females adjust the frequency of feeding their young due to human presence (Lesmerises et al. Reference Lesmerises, Johnson and St‐Laurent2017). Crocodilians, as well as other large predators, are one of the groups that suffer most from the increasing anthropogenic impact, facing several challenges for survival and reproduction (Beal & Rosenblatt Reference Beal and Rosenblatt2020), including behavioural changes (Zhang et al. Reference Zhang, Li, Hu, Ma, Jia, Liu and Nie2023). In this scenario, females are particularly exposed to environmental changes caused by human activities, as they remain close to their nests for extended periods to provide parental care (Vitt & Caldwell Reference Vitt and Caldwell2009). However, little is known about how female crocodilians respond behaviourally to environmental stressors (Hénaut & Charruau Reference Hénaut and Charruau2012).
The simple presence of nests is one of the strongest indicators of reproductive success in crocodilians (Webb Reference Webb2005). The nesting and egg incubation period is one of the most vulnerable stages in the life of crocodilians and therefore requires special care (Mazzotti Reference Mazzotti1989). Massive urbanisation is responsible for the reduction of natural habitats and the increase in negative interactions between humans and crocodilians (Cox & Brumund Reference Cox and Brumund2018; Mascarenhas-Junior et al. Reference Mascarenhas-Junior, Maffei, Muniz, Freitas-Filho, Portelinha, Campos and Bassetti2021). For instance, impacts caused by alterations in natural conditions, such as damming of water bodies, conversion of wetlands to agricultural land, pollution, and mining activities can negatively affect crocodilian populations (Verdade et al. Reference Verdade, Larriera, Piña, Manolis and Stevenson2010; Campos et al. Reference Campos, Muniz, Mourão and Magnusson2020; Mosse et al. Reference Mosse, Kibue and Odadi2023). Under these circumstances, environmental and anthropogenic factors can directly influence characteristics associated with reproduction. Consider the following examples: eggs exposed to pesticides suffer from delays in embryonic development, as well as enzymatic and metabolic disorders (Poletta et al. Reference Poletta, Kleinsorge, Paonessa, Mudry, Larriera and Siroski2011); due to their low selectivity, gill nets inadvertently capture adult females in the reproductive period and even crocodilian hatchlings (Mascarenhas-Júnior et al. Reference Mascarenhas-Junior, dos Anjos, dos Santos and de Sousa Correia2018); habitat degradation causes population decline, reduction in nest density and hatching success (Fujisaki et al. Reference Fujisaki, Rice, Woodward and Percival2007; Mosse et al. Reference Mosse, Kibue and Odadi2023); solid waste present in the environment can be used by females in building nests and negatively affect embryonic development (Barboza et al. Reference Barboza, Correia and Santos2020); gold mining can cause genotoxic effects (Marrugo-Negrete et al. Reference Marrugo-Negrete, Durango-Hernández, Calao-Ramos, Urango-Cárdenas and Díez2019); and fluctuations in incubation temperature interfere with sex ratio, hatching success and hatchling size (Simoncini et al. Reference Simoncini, Leiva, Piña and Cruz2019).
The survival of the young depends heavily on the care females invest in their nests (Mazzotti Reference Mazzotti1989; Lance et al. Reference Lance, Tuberville, Dueck, Holz‐Schietinger, Trosclair, Elsey and Glenn2009). Female crocodilians exhibit strong maternal care, which begins with the careful selection of nesting sites (Royle et al. Reference Royle, Smiseth and Kölliker2012) and extends through the first few months of the juveniles’ life, reducing the vulnerability of eggs and hatchlings to predation (Magnusson Reference Magnusson1980; Murray et al. Reference Murray, Crother and Doody2020). Nesting areas are carefully selected based on a set of variables, such as proximity to water bodies, availability of nesting material, presence and quality of nursery areas (Magnusson Reference Magnusson1980; Somaweera et al. Reference Somaweera, Brien and Shine2013), and solar incidence level (Balaguera-Reina et al. Reference Balaguera-Reina, Venegas-Anaya, Sanjur, Lessios and Densmore2015). The selection of nesting sites by females often leads to intraspecific competition (Cunha et al. Reference Cunha, Barboza and Rebêlo2016; Rodrigues et al. Reference Rodrigues, Barboza, Santos and Correia2021), with larger, older, and more experienced females typically securing the most desirable sites (Montini et al. Reference Montini, Piña, Larriera, Siroski and Verdade2006; Murray et al. Reference Murray, Easter, Merchant, Cooper and Crother2013). Under these circumstances, anthropogenic impacts significantly reduce the availability of suitable nesting areas by causing substantial habitat alteration and degradation (Fujisaki et al. Reference Fujisaki, Rice, Woodward and Percival2007).
Crocodilians of the Caiman genus, particularly C. latirostris (broad-snouted caiman) naturally occur in lentic water bodies along eastern South America in Brazil, Uruguay, Paraguay, Bolivia, and northern Argentina (Coutinho et al. Reference Coutinho, Marioni, Farias, Verdade, Bassetti, Mendonça, Vieira, Magnusson and Campos2013). Their presence has been recorded in large urban centres (Verdade et al. Reference Verdade, Larriera, Piña, Manolis and Stevenson2010; Correia et al. Reference Correia, Maranhão, Mascarenhas Junior, Barboza, Nobrega and Lima2021), exposing them to several anthropogenic factors occurring near water bodies, including pollution by domestic and industrial effluents and solid waste, intense traffic of motorised vessels, hunting and predatory fishing (Neves Reference Neves2019; Barboza et al. Reference Barboza, Correia and Santos2020). Their survival in impacted areas depends largely on the connectivity with the remaining scarce protected areas (Leverington et al. Reference Leverington, Costa, Pavese, Lisle and Hockings2010; Correia et al. Reference Correia, Maranhão, Mascarenhas Junior, Barboza, Nobrega and Lima2021). In light of these challenges, behavioural ecology research plays a crucial role in informing strategic decisions on the welfare and conservation of crocodilians (Caro Reference Caro2007). While studies have sought to assess the impact of anthropogenic factors on various reproductive characteristics of crocodilians, such as the effects of contaminants, degraded areas, human presence, atypical weather events, nutritional stress, and hydroelectric plants (Larriera & Piña Reference Larriera and Piña2000; Beldomenico et al. Reference Beldomenico, Rey, Prado, Villarreal, Munoz-de-Toro and Luque2007; Fujisaki et al. Reference Fujisaki, Rice, Woodward and Percival2007; Simoncini et al. Reference Simoncini, Piña, Cruz and Larriera2011; Fernández et al. Reference Fernández, Simoncini and Dyke2013; Barão-Nóbrega et al. Reference Barão-Nóbrega, Marioni, Dutra-Araújo, Botero-Arias, Nogueira, Magnusson and Da Silveira2016; Campos et al. Reference Campos, Mourão and Magnusson2017, Reference Campos, Muniz and Magnusson2019), the specific impact of these factors on the nesting behaviour of female crocodilians remain unclear. Although each crocodilian species has distinct characteristics, they share ecological nesting and behavioural patterns (Murray et al. Reference Murray, Crother and Doody2020), making C. latirostris a valuable model for addressing and understanding knowledge gaps in other threatened species. Furthermore, behaviour serves as a key indicator for assessing animal welfare, especially in the wild (Dawkins Reference Dawkins2004). In an effort to bridge these gaps, the main objective of this study was to evaluate the relationship between anthropogenic disturbances and nesting characteristics of C. latirostris in its natural environment. To investigate this, we compared nests found in a disturbed area (affected by anthropogenic influence) with nests in a non-disturbed area (free from anthropogenic influence). Given that anthropogenic impacts influence the nesting process, that female crocodilians may change nesting locations (Henaut & Charruau Reference Hénaut and Charruau2012), and that nest density is typically lower in degraded habitats (Fujisaki et al. Reference Fujisaki, Rice, Woodward and Percival2007), we expected to find the following: (i) lower nest density in the disturbed area due to human activity (Eversole et al. Reference Eversole, Henke, Wester, Ballard, Powell and Glasscock2018; Beal & Rosenblatt Reference Beal and Rosenblatt2020); (ii) reduced hatching success in the disturbed area, as documented by Fujisaki et al. (Reference Fujisaki, Rice, Woodward and Percival2007); (iii) a higher proportions of smaller females nesting in the disturbed area, since competition between females for the best nesting areas tends to favour larger, more experienced individuals (Murray et al. Reference Murray, Easter, Merchant, Cooper and Crother2013; Rodrigues et al. Reference Rodrigues, Barboza, Santos and Correia2021); (iv) fewer eggs per nest in the disturbed area, consistent with established allometric relationship between female crocodilians and the size of their clutch (Verdade Reference Verdade2001); (v) lower egg biomass in the disturbed area, reflecting the predominance of smaller females (Murray et al. Reference Murray, Easter, Merchant, Cooper and Crother2013); and finally (vi) a reduction in parental care behaviours in the disturbed area, potentially leading to nest abandonment by females, due to the fact that crocodilians tend to avoid more anthropised environments (Eversole et al. Reference Eversole, Henke, Wester, Ballard, Powell and Glasscock2018; Beal & Rosenblatt Reference Beal and Rosenblatt2020). We are confident that these results will positively impact the welfare and protection of C. latirostris and other crocodilian species.
Materials and methods
Study area and subjects
The study area encompassed the surroundings of the Tapacurá reservoir, built in 1973 in the municipality of São Lourenço da Mata (8º03’S and 35°10’W), in Northeast Brazil (Moura et al. Reference Moura, Azevedo Junior and El-Deir2012). The region is characterised by semi-deciduous forests with a tropical climate, and an annual average rainfall of 1,300 mm (Moura et al. Reference Moura, Azevedo Junior and El-Deir2012). The area includes private properties where agriculture, fishing, and extensive cattle and goat farming are practiced, as well as remaining Atlantic Forest fragments within protected areas with different environmental conservation levels, totalling 590 ha (Figure 1).
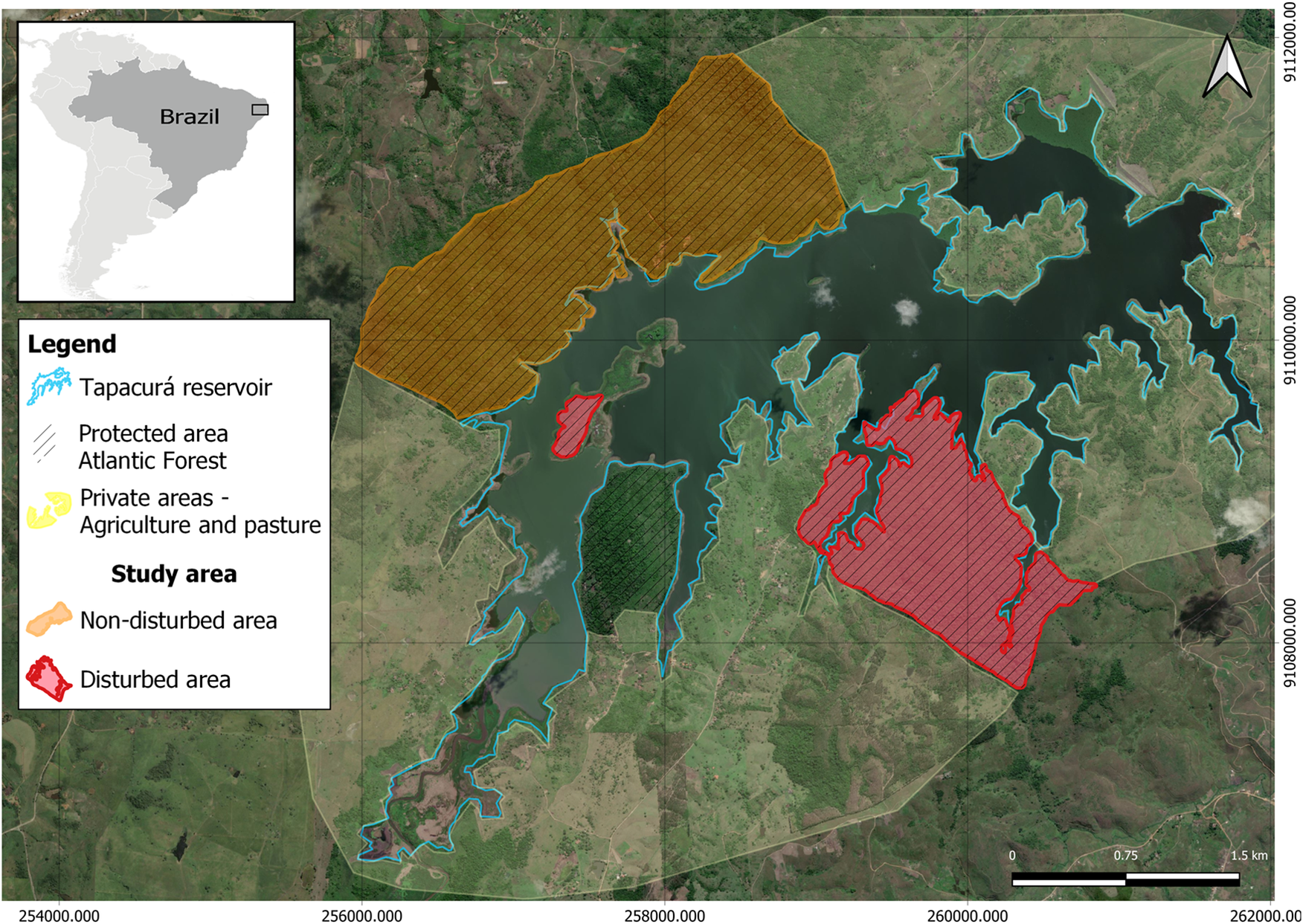
Figure 1. Map of the total area where nests (n = 44) of the broad-snouted caiman (Caiman latirostris) were studied from 2018–2022, showing the different kinds of cover and land usage around Tapacurá reservoir, Pernambuco, Brazil (Bing Satellite images).
The study areas were selected within native forest fragments at various stages of forest maturity. Although the active search method was used in both protected areas, the areas have different surveillance levels, and one of the areas is characterised by intense anthropogenic impact. We categorised the study region into a non-disturbed and a disturbed area based on the level of anthropogenic impact: (i) the non-disturbed area is characterised by ongoing environmental protection (where the presence of humans is limited to surveillance, specific research activities, and environmental education as described by Moura Reference Moura2018), the absence of exotic livestock (cattle or goats), regular daily surveillance to prevent hunting, and native forest fragments at different stages of forest maturity (316.86 ha); (ii) in contrast, the disturbed area lacks consistent environmental protection, with only occasional surveillance, and is characterised by the presence of human activities, unfenced open areas with farmed and domestic animals (cattle, goats, and dogs), and frequent access by people engaged in activities such as plant extraction and hunting (R Barboza, personal observation 2022) (Figure 1). It is worth mentioning that the aquatic environment of the reservoir (710 ha) covers both areas and activities, such as predatory fishing, including illegal caiman hunting, occur there (Mascarenhas-Júnior et al. Reference Mascarenhas-Junior, dos Anjos, dos Santos and de Sousa Correia2018). On the edge of the non-disturbed area, we observed no fishermen, and part of this region is inaccessible due to dense aquatic vegetation (R Barboza, personal observation 2022).
We monitored 44 nests (eight in the disturbed area and 36 in the non-disturbed area), along with the females associated with them. We captured nine of these females, five in the disturbed area and four in the non-disturbed area (Larriera et al. Reference Larriera, Piña, Siroski and Verdade2004), to collect biometric data and mark them for future identification. The females were captured in terrestrial environments by a handler with extensive experience in crocodilian procedures, using a catchpole and appropriate husbandry and handling techniques (Hewitt & Small Reference Hewitt and Small2021). All captured females were released at the capture site after a quick biometric assessment. To minimise stress and ensure positive animal welfare (Kirkwood Reference Kirkwood2013), we avoided capturing females when they could be identified by their scales during nest management. We did not observe any recapture of the same female during the nesting period.
Ethical approval
Research, marking, and capture activities were approved by the Brazilian Environmental Agency (ICMBio/SISBio), under licence #63030-11, and authorised by the Animal Use Ethics Committee (CEUA) #068/2014 and 2022.
Procedures
The study was conducted during the breeding seasons of C. latirostris, from 2018 to 2022. Each field campaign lasted approximately five days, with field trips every two weeks over a four-month period, from February (when nesting and egg-laying occur) to May (when the hatching of the last nest is completed) each year (2018–2022). Nests were found through active daytime searches, covering a 100-m sweep from the waterline at the edge of the reservoir to the interior of the forest. Standardised procedures for field research on crocodilian nests were followed, and data related to the characteristics of the nesting habitat, nests, eggs, females, and hatchlings were gathered (Campos et al. Reference Campos, Muniz and Magnusson2019). The following data were then collected for each sampling area: distance travelled during the active search; the number of nests; the number of eggs per nest (clutch size; CS); egg sizes (length; EL, width; EWI, mass; EMA, and clutch mass; CM); hatching rate (ER; number of hatched eggs/total number of eggs × 100); and female biometric data (snout-vent length; SVL, total length; TL, and body mass; BM). Females were assumed to be the mothers of the nests based on their proximity at the time of capture (within 2–8 m from the nest) and their observed parental care behaviour (Larriera et al. Reference Larriera, Piña, Siroski and Verdade2004). After performing in situ biometric measurements of the egg and females, followed by microchipping and marking through caudal-scale clipping, the females were released at the original capture site (Larriera et al. Reference Larriera, Piña, Siroski and Verdade2004). Viable eggs underwent preliminary identification through the presence of an opaque band of calcification (Brown et al. Reference Brown, Forbes, Myburgh and Nöthling2021).
The presence of the female near the nest after laying was considered evidence of parental care aimed at preventing egg predation (Larriera et al. Reference Larriera, Piña, Siroski and Verdade2004). This information was gathered via direct visual identification of the female at the nest, remote identification using camera traps, or by detecting tracks and traces of nest maintenance throughout the entire monitoring period, from laying to hatching (Barão-Nóbrega et al. Reference Barão-Nóbrega, Marioni, Villamarín, Soares, Magnusson and Da Silveira2014; Merchant et al. Reference Merchant, Savage, Cooper, Slaughter, Perkin and Murray2018). Camera traps (Bushnell Trophy Cam, Bushnell Corporation, USA) were used as a complementary tool to enhance the identification and characterisation of parental care and predation in the absence of human presence. However, we only had access to four cameras, which were installed individually and randomly at different nests in photography mode and moved to a new nest once the eggs were fully predated or hatched. Cameras were installed exclusively in the non-disturbed area due to the risk of theft in the disturbed area.
Statistical analysis
In order to evaluate the influence of the areas (predictor variable) on the nesting characteristics (response variable), the Student’s t-test was performed on the variables that followed a normal distribution (CS and CM), while the Mann-Whitney U test was applied to the variables that did not follow a normal distribution (ER, SVL, and nest density). The Shapiro-Wilk test was used to assess normality. Nest density in each area over five years (number of nests per distance in km covered annually), egg production (CS = total number of eggs per nest), egg biomass (CM = total mass of eggs per nest), and hatching rate (ER = number of hatched eggs/total number of eggs per nest × 100) were analysed to determine the influence of each type of sampled area (non-disturbed vs disturbed). The data collected over the four years of the study were pooled for analysis. A Chi-squared test with Yates correction was used to assess the presence of females performing parental care of the nests in each type of sampled area (Deitz & Hines Reference Deitz and Hines1980).
Data adversely affecting the variables CM, CS, and ER due to predation events (nests lacking information on the total number of eggs), nests with communal nesting (two females laying in the same nest), and eggs not fully separated were excluded. P-values ≤ 0.05 were considered significant.
Results
Over the course of five reproductive seasons, we identified and monitored a total of 44 C. latirostris nests (eight in the disturbed area and 36 in the non-disturbed area). These nests were located throughout a total distance of 58.25 km, with 23 km in the non-disturbed area and 35.25 km in the disturbed area. All the nests were found in forested environments.
Based on the analysed variables, nest density was significantly higher in the non-disturbed area (1.31 nests per linear km; P = 0.009) compared to the disturbed area (0.25 nests per linear km) (Table 1; Figures 2[a] and 3).
Table 1. Analysis of variables related to nesting of Caiman latirostris in the surveyed areas around Tapacurá reservoir, Pernambuco, Brazil, studied from 2018–2022
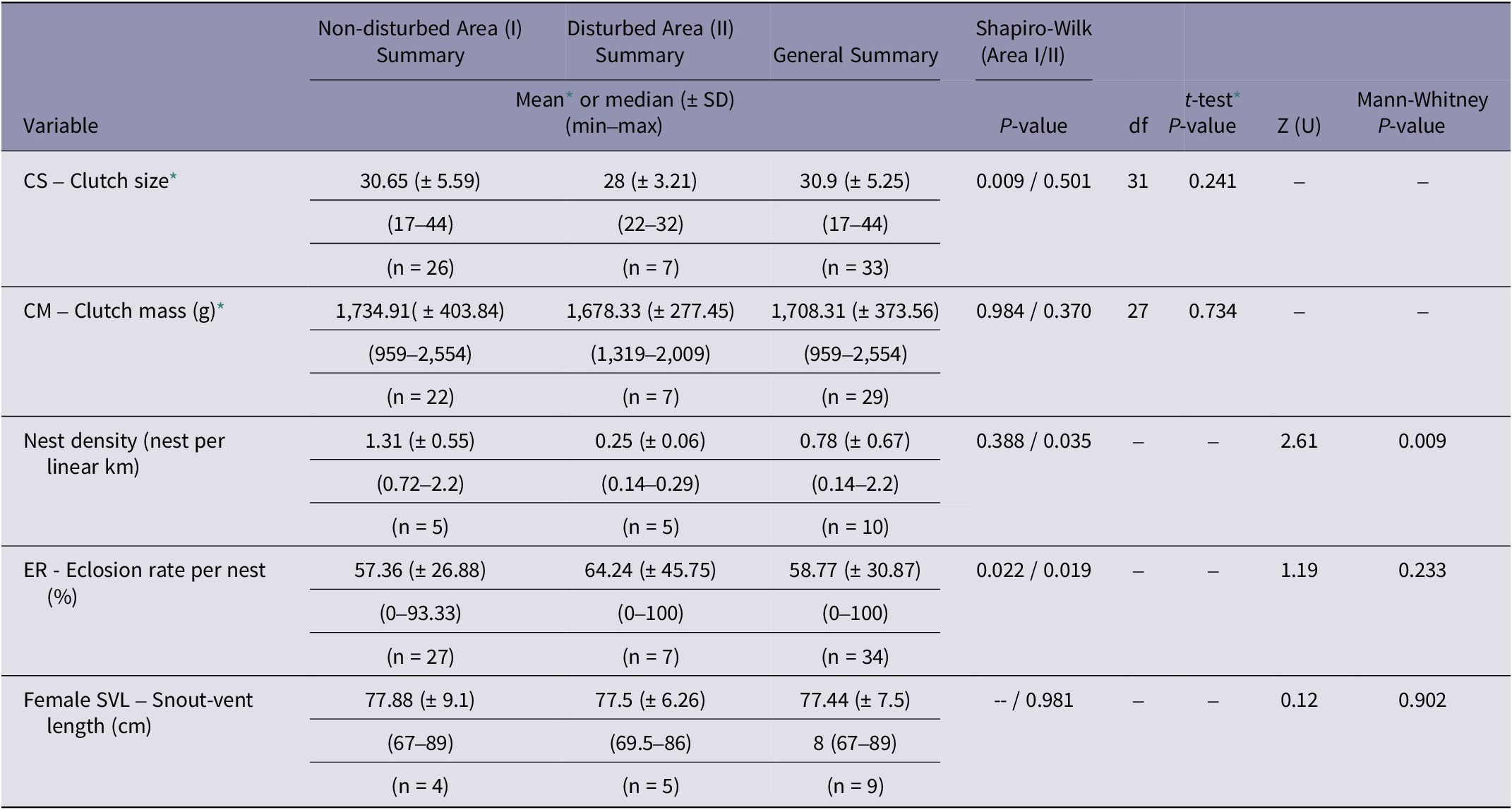
* Data exhibited a normal distribution. df = degrees of freedom.
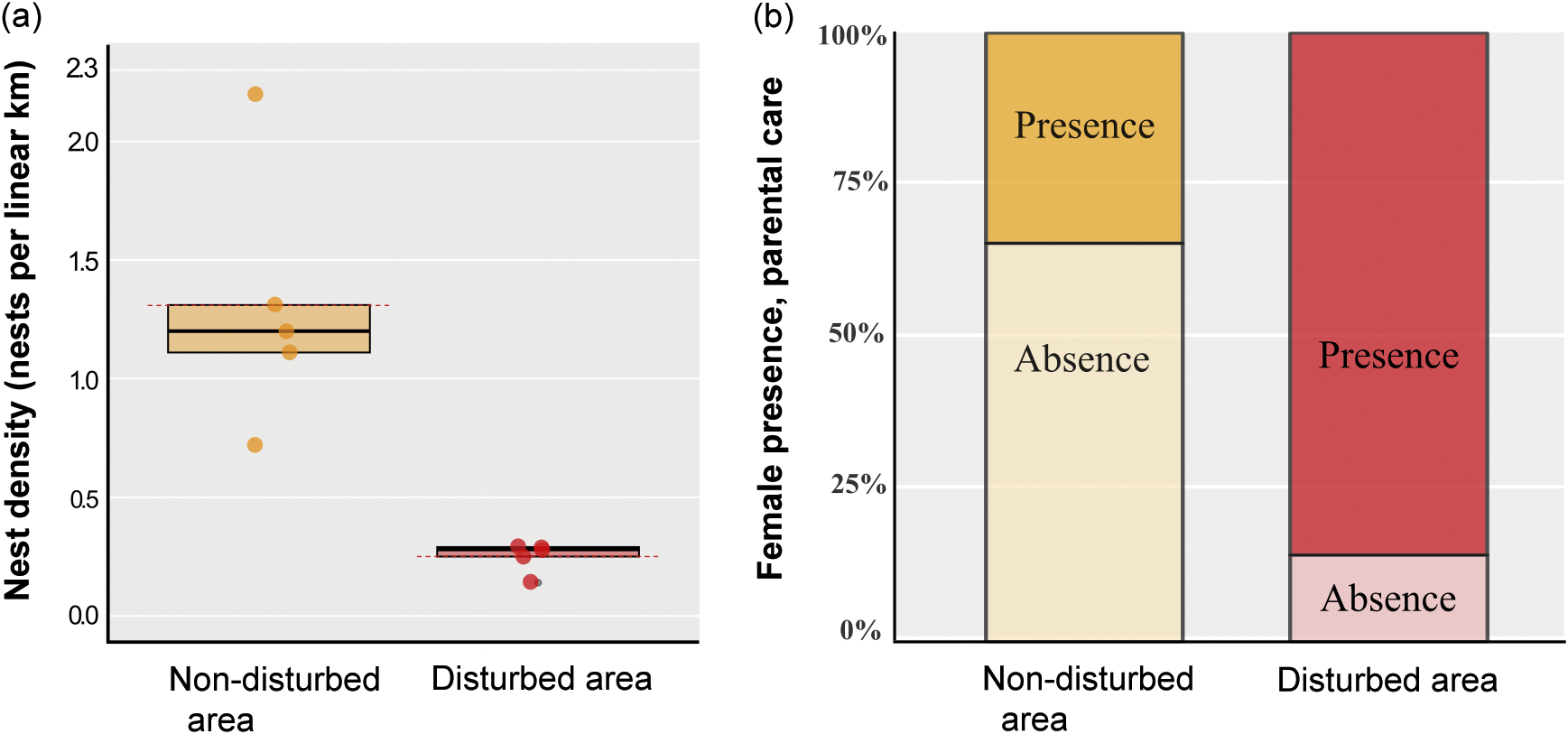
Figure 2. Showing (a) graphic representation of nest density of Caiman latirostris in a disturbed and a non-disturbed area (n = 10; the dashed line represents the mean; * denotes Mann-Whitney; P = 0.009) and (b) representation of the presence of C. latirostris females performing parental care in nests within the disturbed and the non-disturbed area (n = 39; * denotes Chi-squared test; P = 0.04, with Yates correction).
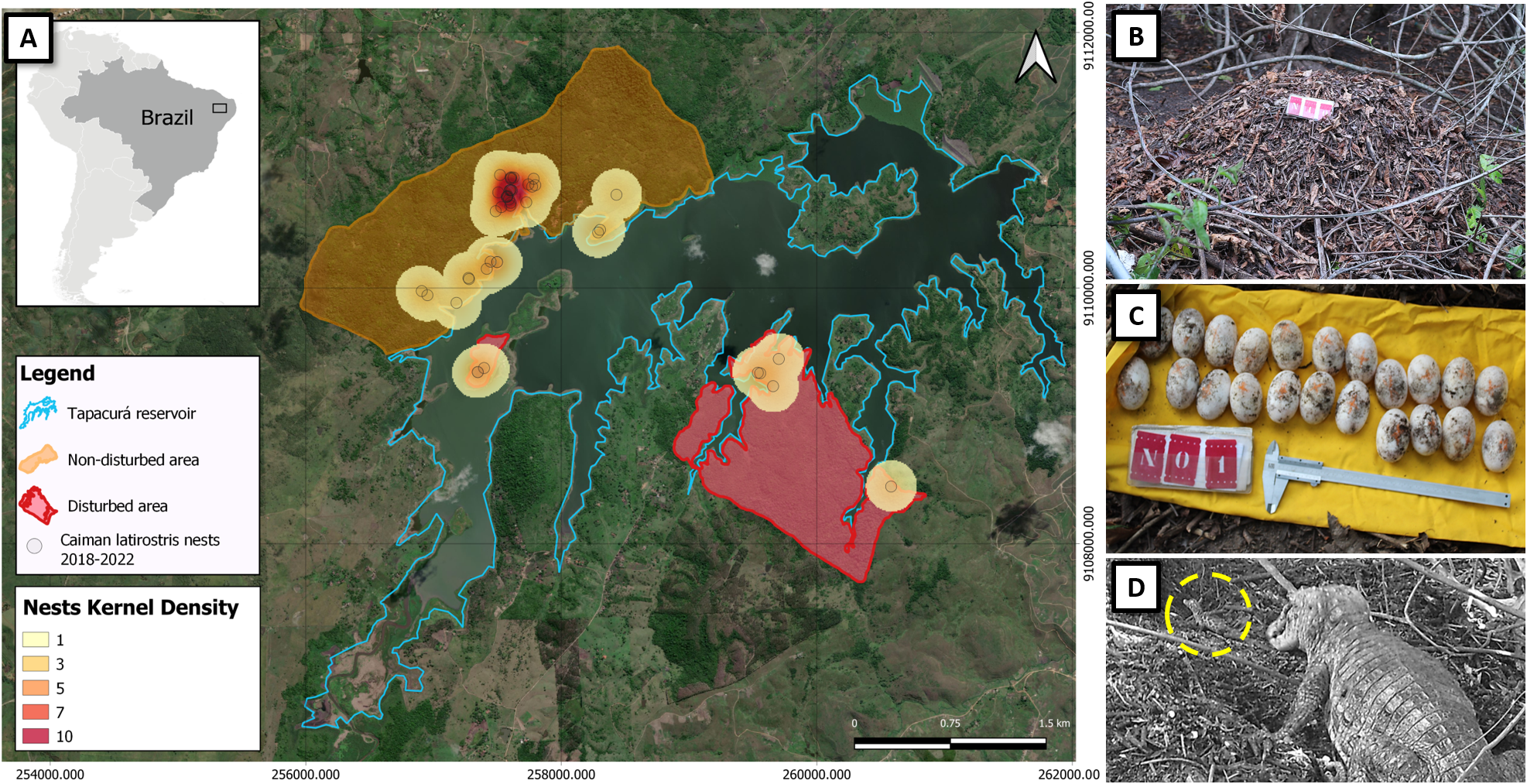
Figure 3. (A) Map showing the spatial distribution of nests (n = 44) of the broad-snouted caiman (Caiman latirostris) in the sample areas studied (2018–2022) around the Tapacurá reservoir, Pernambuco, Brazil (Bing Satellite images). The images depict: (B) a nest, (C) eggs measured for biometrics, and (D) a female assisting a hatchling (dashed circle in yellow), recorded by a camera trap.
Parental care behaviour was significantly higher in the disturbed area (86%) in comparison with the non-disturbed area (34%) (n = 39; df = 1; Chi-squared test; P = 0.04, with Yates correction; Figure 2[b]). No female recaptures were recorded. In the 25 nests equipped with cameras, females were observed engaging in parental care behaviour in only three nests. We documented a range of parental care behaviours, including nest maintenance, nest guarding, nest defence against non-human animals, stillness in the presence of humans, opening nests and aiding in egg hatching, and carrying hatchlings to the water (Figure 3). The other variables, including hatching success, female size, clutch size, and clutch mass did not differ significantly between the two areas (Table 1). Females exhibited an average SVL variation of 77.88 cm, with the smallest nesting female measuring 67 cm in SVL.
Discussion
In the present study, we aimed to assess the influence of anthropogenic activities on aspects related to nesting and parental care in the broad-snouted caiman. To achieve this, we compared a set of variables between an area disturbed by human activity and a non-disturbed area.
We found the average nest density in the disturbed area to be significantly lower than in the non-disturbed area. It is known that crocodilians tend to avoid areas with higher levels of human activity (Eversole et al. Reference Eversole, Henke, Wester, Ballard, Powell and Glasscock2018; Beal & Rosenblatt Reference Beal and Rosenblatt2020), which seems to be consistent with our results. Although some authors consider C. latirostris to be a resilient and tolerant species to anthropogenic pressure (Coutinho et al. Reference Coutinho, Marioni, Farias, Verdade, Bassetti, Mendonça, Vieira, Magnusson and Campos2013), there is evidence of a preference for healthier and more protected environments, especially when selecting nesting sites (Eversole & Henke Reference Eversole and Henke2022). The non-disturbed area in our study site has been subject to increasing conservation efforts since the construction of the dam in the 1970s. It is characterised by restricted land access, comprehensive fencing to control livestock intrusion, Atlantic Forest remnants, reforestation zones, natural landscape recovery areas, and possible human presence excluding any form of extractivism (Moura Reference Moura2018). In addition to the river damming, the increase in water surface area, forest recovery, and limited human access have likely benefited the caiman population by providing more suitable habitats over time. Therefore, non-disturbed sites were identified and progressively used by females for nesting purposes, resulting in a gradual increase in the number of adult females and young in zones considered potential nurseries within this area. Conversely, the open land areas of the reservoir were favoured by large adults (Mascarenhas-Júnior et al. Reference Mascarenhas-Junior, dos Santos, de Moura, Diniz and Correia2020), especially males (Barboza unpublished data). The designated nursery areas were more sheltered aquatic habitats, far from the main body of water, offering food and protection for the young, similar to what was documented in Crocodylus porosus (Magnusson Reference Magnusson1980).
Crocodilians show differences in habitat use between males and females. In Melanosuchus niger, females prefer areas that are difficult to access, particularly near nesting and nursery sites, while adult males tend to inhabit more open areas (Da Silveira & Thorbjarnarson Reference Da Silveira and Thorbjarnarson1999), which is in keeping with our observations in the focal area of our study with C. latirostris (Barboza, unpublished data). Another important factor may be related to preference and intraspecific competition among females. For example, there are records of communal nesting (also see Rodrigues et al. Reference Rodrigues, Barboza, Santos and Correia2021), gregarious nests, and fidelity to nesting sites of C. latirostris within the non-disturbed area (Barboza unpublished data), highlighting the preference for non-disturbed sites. Both sampled areas contain ‘nursery areas’, but nest concentration was lower in the disturbed area. This is probably due to vessel access and predatory fishing activities, which compromise the protection of these nursery areas. Consequently, females may perceive the terrestrial environments near the nests as safer, given the potential risks in the disturbed nursery area. In Alligator mississippiensis, higher nest density is thought to play a key role in promoting hatchling diversity across different clutches (Hunt & Watanabe Reference Hunt and Watanabe1982), which presumably reduces predation risk and enhances parental care efficiency for both eggs and hatchlings in nursery areas.
Contrary to our expectations, there was no significant difference in hatching success between the two types of sampled areas. This is particularly interesting, as one would typically expect reduced hatching success in disturbed areas. However, we observed that females in the disturbed area were more invested in parental care (see below for a more detailed discussion on this topic), which may have contributed to hatching success despite the environmental disturbances. Hatching success is influenced mainly by factors such as egg viability, predation, flooding, and incubation temperature (Campos Reference Campos1993; Simoncini et al. Reference Simoncini, Leiva, Piña and Cruz2019). Embryos can be harmed when eggs are exposed to prolonged sunlight or rotated in predated nests, even if they are not preyed upon directly, further reducing the hatching rate (Donayo et al. Reference Donayo, Piña, Larriera, Verdade and Larriera2002). Importantly, egg loss due to flooding was not observed in the study area, and neither incubation temperature nor predation was assessed due to the risk of camera theft in the disturbed area. Variation in hatching success based on nesting habitat has been documented in other studies of C. latirostris and C. yacare (Campos Reference Campos1993; Imhof et al. Reference Imhof, Costa and Larriera1996; Montini et al. Reference Montini, Piña, Larriera, Siroski and Verdade2006). However, all the nests in this study were located within the same forest habitat. The long-term impact of such behaviour on female welfare remains unanswered.
The hatching success in the study area (59%; Table 1) was similar to that reported in other regions (62% in Argentina: Montini et al. Reference Montini, Piña, Larriera, Siroski and Verdade2006; 66% in Australia: Somaweera & Shine Reference Somaweera and Shine2012). It was also assumed that the larger, more experienced females would prefer nesting in the more protected area (Montini et al. Reference Montini, Piña, Larriera, Siroski and Verdade2006; Murray et al. Reference Murray, Easter, Merchant, Cooper and Crother2013). However, contrary to expectations, we did not find a significant difference between the sampled areas. It is possible that the order of arrival and occupation of the most desirable areas at the beginning of the nesting period had a greater influence than female experience (Simoncini et al. Reference Simoncini, Cruz and Piña2013). The lack of an effect of female size between the sampled areas may also explain the non-significant results observed for the variables egg number and egg biomass. As confirmed in other studies, these variables show positive allometric correlations (Verdade Reference Verdade2001; Larriera et al. Reference Larriera, Piña, Siroski and Verdade2004).
Overall, parental care in the study area was relatively low (43%). Although few studies report similar values, Hunt and Ogden (Reference Hunt and Ogden1991) documented 66% parental care for A. mississippiensis in Okefenokee Swamp, USA. We initially expected that the disturbed area would lead to a reduction in parental care due to the associated impacts and risks. Interestingly, we found the opposite: females nesting in anthropogenic areas displayed significantly more parental care. Thus, the data suggest that females in the disturbed area remain close to the nest from egg-laying until hatching, likely reducing the predation risks and presumably minimising exposure/vulnerability to potential threats resulting from displacements in the water body. This behavioural strategy may reduce energy costs and limit encounters with humans (a potential predator) during the nutritionally demanding period of nest parental care (Barão-Nobrega et al. 2016). On the other hand, the similarity in the results between the two areas for the studied variables (i.e. eclosion rate) may be due to compensation, whereby higher energy costs (Audzijonyte & Richards Reference Audzijonyte and Richards2018) incurred by the females engaging in more intensive parental care in the disturbed area offset the expected differences in hatching success. Nevertheless, it is important to consider that, while maternal care may be an individual behaviour, animals can still display plastic behavioural changes as their initial response to environmental shifts (Tuomainen & Candolin Reference Tuomainen and Candolin2010).
Behavioural adjustments may enhance offspring survival in more disturbed areas (Candolin et al. Reference Candolin, Nieminen and Nyman2014), highlighting the sensitivity of females to anthropogenic disturbances. The only likely predator of adult caimans in our study area is humans, consistent with local reports of frequent predatory hunting and fishing activities (Mascarenhas-Júnior et al. Reference Mascarenhas-Junior, dos Anjos, dos Santos and de Sousa Correia2018; Barboza et al. Reference Barboza, Maranhão, Correia, Barreto-Lima, Santos and Nóbrega2021). This aligns with the absence of natural predators, such as jaguars (Panthera onca), giant otters (Pteronura brasiliensis), and anacondas (Eunectes spp), which are typically the main predators of juvenile and adult caimans (Da Silveira et al. Reference Da Silveira, Ramalho, Thorbjarnarson and Magnusson2010; Ribas et al. Reference Ribas, Damasceno, Magnusson, Leuchtenberger and Mourão2012; Thomas & Allain Reference Thomas and Allain2021). Furthermore, caiman eggs and hatchlings are preyed upon by native species, such as the tegu lizard (Salvator merianae), coati (Nasua nasua), ocelot (Leopardus pardalis), crab-eating fox (Cerdocyon thous), racoon (Procyon cancrivorus) and armadillo (Dasypus novemcinctus) (Barboza et al. Reference Barboza, da Costa, Andrade, de Brito Pezzuti and Rebêlo2012; Oliveira Reference MAB, de Moura GJ and Mendes de Azevêdo Júnior2012), as well as non-native species like domestic dogs (Canis familiaris). Nests can also be trampled by cattle, which have unrestricted access to the disturbed area. Several of these animals were observed near the nests, captured by camera traps, and have been described in previous studies (Moura et al. Reference Moura, Azevedo Junior and El-Deir2012).
Our findings suggest that it is crucial to address the impact of researchers’ presence on the parental care behaviour of females. With camera traps, we identified and recorded parental care behaviours of five females, who had no direct contact with the research team during the same reproductive season of the photographic record. These females, recorded by camera traps, were observed engaging in activities such as chasing away predators (e.g. tegu lizard), maintaining nests that had been disturbed by predators, opening nests to help hatchlings, and transporting them to the water. However, among the nine captured females, two were present at the nests but were not captured by the researchers on two separate occasions. These brief interactions with the team were due to the need to adjust the camera near the nest. Nevertheless, it appears that both females abandoned their nests shortly afterwards, as no further evidence or traces of their presence were found. The eggs from these nests were not predated. During the encounters, all females displayed inert behaviour, indicating caution towards human presence, which was also documented for A. mississippiensis by Kushlan and Kushlan (Reference Kushlan and Kushlan1980). Some researchers claim that females’ lack of aggressive behaviour towards humans near nests may result from negative past experiences, given that crocodilians can learn (Bustard Reference Bustard1968; Joanen & McNease Reference Joanen and McNease1989; Webb & Messel Reference Webb and Messel1979; Somaweera et al. Reference Somaweera, Webb, Brown and Shine2011; Hénaut & Charruau Reference Hénaut and Charruau2012). The tendency for parental care abandonment observed in response to the mere presence of humans in our study suggests that these animals perceive humans as potential threats. This perception likely stems from previous negative experiences and the risks associated with human activity in the reservoir area (i.e. hunting; Mascarenhas-Junior et al. Reference Mascarenhas-Junior, Strickland, Heithaus, Santos, Barboza, Simões and Correia2024). As early as 1980, Kushlan and Kushlan also reported that female crocodilians recognise humans as potential threats and may alter their behaviour. Other studies also indicate that human disturbance or capture of females, even during research, can cause them to leave the nest (Staton & Dixon Reference Staton and Dixon1977; Deitz & Hines Reference Deitz and Hines1980; Magnusson Reference Magnusson1980, Reference Magnusson1982; Mazzotti Reference Mazzotti1989; Hunt & Ogden Reference Hunt and Ogden1991; Lance et al. Reference Lance, Tuberville, Dueck, Holz‐Schietinger, Trosclair, Elsey and Glenn2009) or to change nesting sites in subsequent years (Mazzotti Reference Mazzotti1989). In contrast, Barão-Nóbrega et al. (Reference Barão-Nóbrega, Marioni, Villamarín, Soares, Magnusson and Da Silveira2014) and Simoncini et al. (Reference Simoncini, Marcó, Portelinha and Piña2016) documented that researchers’ activity at the nests did not influence the parental care behaviour of females, which differs from our findings.
Animal welfare implications
This study underscores the importance of preserving non-disturbed habitats for the welfare and protection of crocodilian species and provides valuable insights into their behavioural responses to anthropogenic disturbances. Especially for crocodilians, parental care is a crucial strategy to increase the chances of survival, reproductive success, and the development of young recruits. Disruptions to this process can adversely affect the welfare of females and, consequently, their nest protection behaviour. Further research is needed to fully understand the long-term effects of anthropogenic activities on crocodilian populations and to develop effective welfare and conservation strategies. Additionally, research on the impact of human disturbance on parental care behaviour should be extended to include other variables, crocodilian species, and geographic regions to better inform welfare and conservation practices.
Conclusion
In general, the awareness of crocodilian females, especially during parental care, should not be underestimated. Behavioural choices and traits suggest that these animals may not be as resilient to anthropogenic pressures as previously thought. Their preference for more pristine nesting sites indicates that anthropogenic disturbances must be seriously factored in to develop effective welfare and conservation strategies. This is particularly important if we consider that, despite a preference for the non-disturbed area, disturbed sites are also used for nesting. Moreover, increased nest parental care in the disturbed area indicates that the females are aware of the additional risks this area poses. We further suggest that direct human-caiman interaction is a critical factor that should not be overlooked, as it may interfere with parental care behaviour.
Although this study provides valuable insights into how human disturbances affect nesting behaviour and parental care in C. latirostris females, it is important to account for unanalysed variables that may also influence these behaviours and warrant investigation in future studies. Differences between the surveyed areas may reflect significant selective pressures affecting behaviours, such as nest site selection and female presence at nesting sites. Therefore, considering disturbance levels as the primary factor influencing behaviour demands a careful interpretation of field findings. Further research is needed to better understand if the behaviours observed in C. latirostris females in our study occur in other crocodilian species. Given their cryptic nature, we emphasise the importance of implementing increasingly non-invasive research methods using alternative technologies to minimise or eliminate interference with their behaviour and promote better welfare (e.g. Kirkwood Reference Kirkwood2013; De Moraes et al. Reference De Moraes, Da Silva Souto and Schiel2014; González-Desales et al. Reference González-Desales, Tello-Sahagún, Cadena-Ramírez, López-Luna, Buenrostro-Silva, García-Grajales, González-Ramón, Morales-Mavil, Charruau, Sigler, Rubio-Delgado, Zarco-González and Monroy-Vilchis2020).
A key step for future longitudinal studies is to investigate the survival of newborns in areas with varying levels of disturbance. Overall, it is imperative to foster welfare and conservation efforts by preventing predatory hunting and fishing of caimans, as well as by identifying and protecting nursery areas. Preserving terrestrial and aquatic ecosystems is an effective strategy for the welfare and conservation of this animal group. Establishing a population and reproductive monitoring programme will help evaluate the effectiveness of the adopted conservation strategies. We are confident that these actions will contribute to supporting the welfare and conservation of C. latirostris and other crocodilian species.
Competing interest
None.






