Introduction
With an estimated 1.75 million deaths from cancer in Europe in 2012 (Reference FerlayFerlay et al., 2013), cancer is the second leading cause of death in Europe (World Health Organization, 2012). Diagnosis, treatment, continuing care, and in many cases palliation account for a substantial volume of the work of the acute general hospital. Yet while the word “cancer” is widely used in popular discourse, it is important to recognize that it is not a single disease but rather a pathological process that can affect almost all organs of the body. This process involves uncontrolled tissue growth, based on changes related to genetic or acquired abnormalities of the DNA, or related processes in the cell. Genetic changes that cause cancer can be inherited or, more commonly, they arise during a person’s lifetime as a result of errors that occur by chance as cells divide or because of damage to genes caused by environmental exposures such as chemicals in tobacco smoke or ultraviolet radiation. The clinical management of cancer thus depends on both the nature of the pathological processes involved, increasingly being characterized at the molecular level, and the organs affected. An individual’s cancer is thus characterized by a unique combination of genetic changes, which can change over time, for instance as a result of developing resistance by selecting clones of therapy-resistant cells. As a consequence, there has been an important paradigm shift from organ-based interventions to a patient-centred and targeted treatment for which increasingly innovative biological therapies are being discovered. In addition, cancer shares certain risk factors with other diseases. Thus, patients with cancer may be at greater risk of those other conditions. For example, tobacco use, which is the leading preventable cause of cancer in Europe, is associated not only with cancers of the lung but also with many other cancers, while also contributing to other conditions, such as coronary heart disease (Reference PetoPeto et al., 2012).
Cancer diagnosis and treatment have changed substantially in the past decades, with, for example, the advent of new chemotherapeutic agents transforming many cancers from short-lasting fatal illnesses into long-term chronic disorders. With increased understanding of the underlying disease processes, there have been considerable advances in early detection and diagnostic imaging, genetic profiling, and increased treatment options, including the introduction of targeted drugs and multidisciplinary care in many settings. In addition, and related to improved survival rates, cancer care takes account of psychosocial aspects, quality of life, patients’ rights, and empowerment and survivorship. In this chapter we explore these shifts in cancer treatment and care, with a focus on oncological hospital care in Europe.
The burden of cancer in Europe
In 2008 one-quarter of the global cancer burden was observed in Europe, which is striking given that the total European population comprises only one-ninth of the world’s population (Reference FerlayFerlay et al., 2013). For 2012 the Globocan project predicted an incidence of 3 715 000 cases with a five-year prevalence of 9 701 000 cases. There were an estimated 3.45 million new cases of cancer (excluding non-melanoma skin cancer) and 1.75 million deaths from cancer in Europe in 2012 (Reference FerlayFerlay et al., 2013).
Female breast cancer (464 000 cases), colorectal cancer (447 000), prostate cancer (417 000) and lung cancer (410 000) were the most frequent cancers, together representing half of the overall cancer burden in Europe in 2012 (Reference FerlayFerlay et al., 2013). The most common cancer modalities leading to death in 2012 were lung cancer (353 000 deaths), colorectal cancer (215 000), breast cancer (131 000) and stomach cancer (107 000). Incidence varies across the region, however, with cancers resulting from external carcinogens and bacteria (e.g. stomach cancer) tending to be higher in eastern Europe and Portugal, while breast and prostate cancer are more common in western Europe. Data from the International Agency for Research on Cancer (IARC), an agency within the World Health Organization, found the incidence in western European countries to be over 244 per 100 000 in 2012, compared to between 177.3 and 244.2 per 100 000 in eastern Europe.
Overall, cancer incidence continues to rise, with growth rates of up to 2% or 3% per year across Europe. As survival is also gradually improving, prevalence is growing at an even quicker pace, leading to increased numbers of cancer patients (those still alive but in various stages of disease after primary treatment) and cancer survivors (those who have ended therapy and are in follow-up schedules).
Table 5.1 Summary indicators of cancer burden in selected high income regions, 2012
| North America | EU-28 | Western Europe | Northern Europe | Southern Europe | Australia and New Zealand | |
|---|---|---|---|---|---|---|
| New cancer cases | ||||||
| Age-standardized rate (per 100 000) | 315.6 | 273.5 | 298.7 | 277.4 | 253.6 | 318.5 |
| Risk of getting cancer before age 75 (%) | 30.9 | 27.3 | 29.6 | 27.5 | 25.3 | 30.7 |
| Cancer deaths | ||||||
| Age-standardized rate (per 100 000) | 105.5 | 109.4 | 105.0 | 108.0 | 105.2 | 97.6 |
| Risk of dying from cancer before age 75 (%) | 11.2 | 11.5 | 11.0 | 11.2 | 10.9 | 9.9 |
| Five-year prevalent cases, adults (per 100 000) | 1 888.2 | 1 690.4 | 2 018.6 | 1 658.6 | 1 585.3 | 1 901.8 |
| Five most frequent cancers (defined by total number of cases) | Prostate Breast Lung Colorectal Bladder | Breast Prostate Colorectal Lung Bladder | Prostate Breast Colorectal Lung Bladder | Prostate Breast Colorectal Lung Melanoma of skin | Colorectal Breast Lung Prostate Bladder | Prostate Colorectal Breast Melanoma of skin Lung |
Note: Estimates of worldwide age-standardized incidence and mortality as provided by GLOBOCAN use the World standard population, while EUCAN uses the European standard population. The World standard population presents a young population compared to the European standard population; EUCAN estimates for individual European countries or regions (such as those reported by Reference FerlayFerlay et al., 2013) are therefore higher than those provided by GLOBOCAN.
Cancer survival rates are typically used as an indicator of the quality of cancer care, from prevention and screening to treatment. In Europe the EUROCARE study has systematically collected survival data from national cancer registries to monitor trends in cancer survival in children and adults (Reference BerrinoBerrino et al., 2007; Reference AngelisDe Angelis et al., 2014). EUROCARE data show large differences in survival, with some analyses linking differences in survival to differences in spending on cancer care, with the Nordic countries and Switzerland scoring favourably compared to the rest of Europe (Reference Luengo-FernandezLuengo-Fernandez et al., 2013). However, interpretation of the data is challenging because of persisting variations in the quality of data available, and the challenges of adjusting for case mix-when interpreting observational studies (Reference LymanLyman, 2013), as well as how best to respond to this evidence (Reference WhalenWhalen, 2010). What is clear is that greater resources will be needed to respond to the combination of a projected rise in the number of cancer cases and technological innovations that could potentially improve outcomes (Reference Aggarwal, Ginsburg and FojoAggarwal, Ginsburg & Fojo, 2014).
The development of contemporary cancer care
Cancer diagnosis and treatment have progressed rapidly since the discovery of the cellular origins of cancer in the 1860s, but mainly in incremental steps. Important developments came about from the 1950s onwards, with advances in radiation technology and, in particular, cancer chemotherapy such as the treatment of childhood leukaemia and, in adults, Hodgkin’s disease from the mid-1960s, with success of adjuvant treatment of breast cancer since the 1970s (Reference DeVita and RosenbergDeVita & Rosenberg, 2012). At the same time, a greater understanding of the causes of cancer has increased scope for primary prevention, reducing the risk of developing disease. The most prominent example is perhaps the discovery of tobacco smoking as a cause of cancers of the lung and various other organs, with declines in the occurrence of lung cancer and subsequently mortality as a consequence of antismoking measures (Reference Jha and PetoJha & Peto, 2014). The discovery of certain viral infections as a cause of cancer has also led to the development of vaccines against, for example, certain types of human papilloma virus (HPV) for the prevention of cervical cancer or hepatitis B virus for liver cancer.
Recent advances in the understanding of biological functioning of the cell have increased our understanding of cellular mechanisms, enabling the development of targeted drugs that have been very successful in certain subgroups of patients. Among the most recent developments is immunotherapy, which provides for progression-free survival in tumours that were previously considered to be uniformly fatal, such as metastatic melanoma and lung cancer.
While advances in screening have enabled early detection of certain cancers, albeit at a risk of over diagnosis for some (Reference ViguierViguier, 2015), these changes have had considerable implications for the management of cancer, which increasingly involves a complex array of interventions that require different professionals working together in a coordinated fashion to enhance outcomes for people with cancer. As it can involve many disciplines, sequential and parallel process steps, different handovers and frequent patient contacts by different disciplines, cancer care is increasingly organized through MDTs and along cancer care pathways.
The cancer care pathway
The cancer care pathway describes the patient’s journey from the initial suspicion of cancer and symptom-based investigations, or through screening and early detection, to the various diagnostic procedures leading to a diagnosis of cancer, followed by treatment, which typically involves a selection of one or more interventions, such as surgery, radiotherapy, or chemotherapy. Depending on the outcome of the primary treatment, the patient will receive follow-up care and rehabilitation or, where the tumour remains active or is advanced, undergo further treatment or receive palliative and end life care when the tumour proves incurable (Figure 5.1).
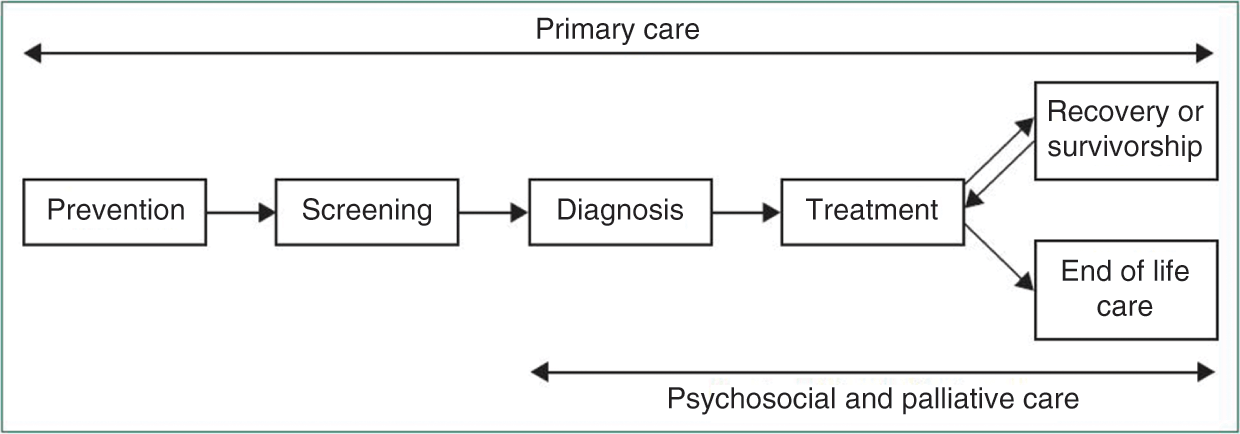
Figure 5.1 The cancer journey
The precise nature and scope of the cancer care pathway differs between cancer types and countries. Detailing cancer care pathways provides patients and professionals with a better understanding of the complex processes that are involved in treatment, while also contributing to enhancing the patient journey to strengthen high quality cancer care. Each pathway identifies the different steps and recommended care processes at each stage of the journey (Cancer Council Australia, 2016).
In countries where the general practitioner (GP) acts as gatekeeper to specialist care, such as the United Kingdom and the Netherlands, most cancers are diagnosed by a specialist after presentation of complaints or symptoms to the patient’s GP, while others are diagnosed upon emergency presentation (Reference RubinRubin et al., 2015). A proportion of cancers is detected through screening programmes such as for breast cancer, colorectal and cervical cancer, although percentages differ across cancer sites and coverage of related programmes in different health systems. Countries where patients can directly access specialist care enable direct and extensive diagnostics; this can lead to overuse of diagnostic procedures. On the other hand, there are some concerns that overly stringent primary care gatekeeping may introduce inappropriate delays. Clearly, it is difficult to get the right balance.
As we shall see below, the diagnosis and treatment of common tumour types is commonly provided by medical specialists within a hospital setting. Many countries have also established designated cancer centres for the delivery of specialized care for a large portfolio of common and rare tumours, serving also as tertiary referral centres for patients with rare tumours, late-stage disease or other difficult cases. Comprehensive cancer centres usually undertake a wide range of activities in translational cancer research, from basic scientific discovery, to the delivery of novel approaches, to care of patients with cancer, such as targeted therapies.
The precise nature of the cancer patient journey differs for different types of cancer. Figure 5.2 illustrates a typical pathway for a patient with breast cancer in a cancer centre in the Netherlands. In this example, the patient would typically consult with a range of specialists within the cancer centre, which would also be responsible for aftercare. In some countries therapy is provided in “shared care” arrangements involving office-based physicians or local hospitals, for instance in order to provide chemotherapy closer to the patient’s home.

Figure 5.2 Breast cancer patient pathway, the Netherlands
Although most types of cancer care require specialized equipment and staff, there is an increasing trend to move more parts of the cancer care pathway into the community. Care in the community takes several forms, including chemotherapy delivered in people’s own homes (Reference CorbettCorbett et al., 2015), rehabilitation in community settings, blood and other monitoring tests in general practices or local settings, and increased access to local services and support groups (Macmillan, 2014).
Patient-focused, integrated care initiatives can provide greater quality, efficiency and patient satisfaction (Reference LeutzLeutz, 1999; Reference Burns and PaulyBurns & Pauly, 2002; Reference Kodner and SpreeuwenbergKodner & Spreeuwenberg, 2002). Evidence emerging over the past 20 years suggests that the transition of cancer care from oncologist-led models to nurse-led models in cancer centres or primary care-led models in the community may improve cancer outcomes (Reference GrunfeldGrunfeld et al., 1999; Reference WattchowWattchow et al., 2006; Reference LewisLewis et al., 2009; Reference Grunfeld and EarleGrunfeld & Earle, 2010; Reference SussmanSussman et al., 2011). Primary care providers are often willing to assume follow-up care with appropriate guidance and a clear path for transition of care for their patients, and they are more likely than oncologists to provide preventive interventions directed at non-cancer conditions (Reference GiudiceDel Giudice et al., 2009; Reference Grunfeld and EarleGrunfeld & Earle, 2010).
Multidisciplinary teams
We have noted above that the management of cancer increasingly involves a complex array of interventions that require different professionals working together in a coordinated fashion to enhance outcomes for people with cancer. Figure 5.3 provides an illustration of the range of staff involved in the breast cancer care pathway introduced in Figure 5.2. Optimizing delivery of the care pathway and patient outcomes will require close coordination and communication among the different professionals involved. Health providers are increasingly drawing on MDT working to enhance decision-making between health care team members and patients (Reference FleissigFleissig et al., 2006; Reference BorrasBorras et al., 2014). MDTs usually address one type of cancer or a group of cancers. Oncology MDTs can include surgeons, diagnostic and therapeutic radiologists, pathologists, medical and clinical oncologists, nurse specialists, and palliative-care physicians, among others. Such a team will often collaborate closely with other supportive professionals, such as psychologists and psychiatrists (Reference FleissigFleissig et al., 2006).
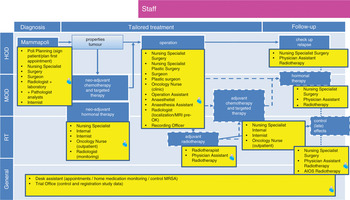
Figure 5.3 Breast cancer pathway for staff, the Netherlands
The move towards MDT working in oncology has been supported by several expert groups and it is now considered the standard in cancer care in most countries in Europe and elsewhere (Reference BorrasBorras et al., 2014). Some countries require that the management of all patients with cancer within MDT conferences should be the norm, although some cases that are uncomplicated will be dealt with according to standard guidelines without being discussed by all involved.
Overall, the adoption of MDTs in cancer care has been rapid. For example, in England in the mid-1990s fewer than 20% of cancer patients were managed by an MDT compared with more than 80% in 2004 (Reference Griffith and TurnerGriffith & Turner, 2004). In the Netherlands the peer review system for hospital cancer services, which was introduced in 1994, provided a strong stimulus for the adoption of MDT working (Reference KilsdonkKilsdonk et al., 2015a, Reference Kilsdonk2015b). Although it is difficult to evaluate the exact mechanism through which an MDT exerts its effect and little direct evidence exists, MDT working has been linked to improved patient outcomes, increased recruitment into clinical trials, and better job satisfaction and psychological well-being among health professionals (Reference FleissigFleissig et al., 2006; Reference PillayPillay et al., 2016). For example, Reference PillayPillay et al. (2016) found, based on a systematic review, that MDT meetings impact positively upon the ways cancer patients are assessed and managed. This is consistent with a review by Reference TaplinTaplin et al. (2015), which suggests that using team-based approaches across the care continuum can improve access to and the quality of care processes and structures. However, robust evidence on the impact of MDTs on patient outcomes such as survival remains weak. Overall, while they appear intuitively to be beneficial, the current evidence base provides only a limited degree of support for their widespread use (Reference PillayPillay et al., 2016) and, at least for now, it may be more cost-effective to limit MDT meetings to the discussion of particularly complex or controversial patients.
Barriers to delivering optimal care and the sustainability of the oncological service system
Workforce
The hospital workforce more broadly, and the oncology workforce specifically, face several barriers regarding the delivery of optimal and sustainable cancer care. Key challenges include demographic changes in the composition of the workforce, including an ageing health workforce, leading to shortages due to retirement (European Commission, 2008), along with fewer younger generations entering the workforce due to the limited attractiveness of employment in the health sector (European Commission, 2008). Further challenges are related to the mobility of the workforce across the EU, in particular the movement of some health professionals from poorer to richer countries within the EU, as well as the health brain drain from third countries (European Commission, 2008).
Expensive biological drugs
A review of market access to cancer drugs in Europe found that reimbursement mechanisms, the use of cost-effectiveness analysis in decision-making, and the extent of pharmaceutical price regulation schemes vary considerably across countries (Reference PauwelsPauwels et al., 2014; Reference Hartenvan Harten et al., 2016). Most countries have some form of risk-sharing agreement for high value drugs, be it financial agreements where rebates are offered to third-party payers for the cost of increased expenditure over an annual subsidization cap, or performance or outcome-based agreements (Reference CheemaCheema et al., 2012).
Overall, there are marked differences in the availability and reimbursement of new and often expensive cancer drugs. For example, in Italy innovative new cancer drugs are classified as Class H, qualifying their use in the hospital setting. Class H drugs are bought directly by hospitals from the manufacturers, enabling them to benefit directly from cost sharing agreements and minimum discounts of 50%. This has enabled expansion of patient access to pharmaceuticals (Reference Folino-GalloFolino-Gallo et al., 2008). In the United Kingdom the National Institute of Health and Care Excellence (NICE) provides advice on whether or not to reimburse innovative drugs; in other countries there are comparable agencies although the implications of their decisions vary. Differences
in the availability of cancer medicines across countries, and the cost of cancer treatment, have prompted considerable public debate in a number of countries. However, the impacts of variation in access to innovative medicines on cancer outcomes at population level are difficult to ascertain.
Radiotherapy and radiology
Unlike cancer drugs, the evaluation of radiation technologies has attracted less attention although it is an area that has undergone significant development over the past 5 to 10 years. Radiotherapy is considered a necessary component of treatment in about half of all newly diagnosed cancers (Reference DelaneyDelaney et al., 2005). However, European countries are in the paradoxical situation where delivering affordable radiotherapy over the next 20 years is being compromised by both current under-capacity and under-investment in “standard” radiotherapy and also over-penetration of newer radiotherapy technologies that have far greater associated costs (Reference Van LoonVan Loon et al., 2012). A recent analysis of the Directory of Radiotherapy Centres (DIRAC) database demonstrated variation in radiotherapy capacity and quality across the EU (Reference RosenblattRosenblatt et al., 2013).
Imaging techniques and radiology play a major role in the management of many patients, including cancer patients (see Chapter 9). The quality of imaging has improved significantly over recent decades and the use of these new devices has increased, although often because of a belief – not always justified – that “newer is better” (Reference DeyoDeyo, 2002). However, the greater use of these techniques has created a larger problem. They often lead to diagnosis of lesions of dubious clinical significance (Reference LumbrerasLumbreras et al., 2010).
An unexpected finding can trigger additional medical care, including unnecessary tests and other diagnostic procedures and treatments which, in some cases, may pose an additional risk to the patient. This process has been called the “cascade effect” (Reference WhitingWhiting et al., 2003). A review by Reference LumbrerasLumbreras et al. (2010) found that a considerable percentage of patients in whom incidental findings were observed underwent further evaluation with additional expensive and often uncomfortable or risky imaging tests or other diagnostic tests and procedures. Radiologists and clinicians have to balance the diagnostic potential against unnecessary testing and treatment (Reference LumbrerasLumbreras et al., 2010). Some measures have been recommended to clarify the situation (Reference WhitingWhiting et al., 2003), including explicit assessment of the potential risk of the incidental finding for the patient or the availability of a beneficial treatment that justifies follow-up, although the optimum strategy will depend greatly on the particular circumstances.
General trends in oncology care
Looking ahead, there are some trends in cancer care that will have especially profound consequences for the hospital. These include precision medicine, targeted treatment, and immunotherapy; image guided interventions; and improved survivorship and survivorship care.
Precision medicine, targeted treatments, and immunotherapy
Greater understanding of the mechanisms by which cancers develop has provided important insights into interventions targeting under-
lying mechanisms and treating the condition (Reference SagerSager, 1997). The primary treatment option is removing the tumour through surgical or radiotherapeutic intervention (often accompanied), which remains by far the most common treatment by which patients are cured (World Health Organization, 2016). However, a considerable percentage of patients are not cured or experience relapse or metastatic disease. Here, chemotherapy and the more recently developed targeted treatments provide important therapeutic options.
Until recently, chemotherapy was given to a large number of patients on the understanding that only a certain percentage would benefit. A better understanding of the underlying pathways has helped to develop treatments that target mechanisms acting at cellular, subcellular or molecular levels. These targeted treatments rely on molecular diagnostics of underlying cell abnormalities, and expertise in genetic aberrations of tumours (Reference GingerasGingeras et al., 2005). Targeted therapies have shown promising results in a number of tumours, especially in advanced stages where so far very few therapeutic options were otherwise available, requiring genome sequencing and analytical techniques along with professional expertise to interpret and weigh findings. It is expected that this trend towards precision medicine, which includes health care innovations involving molecular diagnostics and pharmacogenomics, will continue to bring promising results. This is expected to generate a rapidly growing industry in which genetic markers of disease and treatment responses are searched on a larger scale (Reference DzauDzau et al., 2015), but which could come at a price that can threaten the financial sustainability of health systems.
In recent years immunotherapy (also referred to as biological therapy) has been shown to be promising in treating certain cancers (and other diseases). This was informed by the observation of “spontaneous” cures in some patients, stimulating research into immune reactions around and inside tumours (as, for instance, observed by white blood cell activity). Immunotherapeutic drug options are available for (metastasized) lung and renal cancer and melanoma, with further experimentation under way with other tumour types. DNA vaccination, stimulating antitumour cell reaction, is also being tested (Reference StockwellStockwell, 2015; Reference BlankBlank et al., 2016) . It is estimated that up to 20% of tumours may benefit from some form of immunotherapy in future. This method has the potential to provide treatment for patients who until recently have had no curative options. However, the costs involved have meant that there is considerable variation in access to new immunotherapeutic drugs and treatments across countries, generating debate on pricing levels and sustainability of the financing of cancer treatment. Recent studies have shown marked differences in list prices and actual prices in a number of European countries; overall access to innovative drug treatment in cancer seems especially difficult in the less developed economies in Europe (Reference JohnssonJohnsson et al., 2016; Reference HartenVan Harten et al., 2016; Reference Vogler, Vitry and BabarVogler, Vitry & Babar, 2016).
Image guided interventions
Surgical and radiotherapeutic removal of the tumour (bulk) tissue are the primary treatment options if curative treatment is considered. Complete removal is essential but is not always successful. For example, a study on prostatectomy showed that 38% of patients had not had the tumour tissue completely removed (Reference RetelRetel et al., 2014). Here, imaging guidance, a technique available in fields such as cardiology and neurosurgery, has become an important aide to distinguish normal from malignant tissue, or where the tumour is hard to delineate from the surrounding environment. These include perioperative CT scanning, smart needles with optical features and navigation technology combined with image integration. These methods require expertise and infrastructure in imaging modalities as well as biomedical technology expertise within the operating theatre (see Chapter 9). The investments related to these developments can lead to the gradual concentration of diagnostic and intervention infrastructure and expertise. Most technologies are in “proof of principle” or early phased clinical studies and in oncology in innovator locations, such as the Netherlands Cancer Institute in Amsterdam and the Institut Gustaphe Roussy in Paris.
Improved survivorship and survivorship care
Improved survival rates (Reference StockwellStockwell, 2015) have led to a continuously growing number of cancer patients who have survived primary treatment but require ongoing treatment, including cancer survivors treated with curative intent, and who require follow-up and symptom-related aftercare (Reference Van HartenVan Harten et al., 2013).
Improved cancer survivorship poses challenges for health services, requiring a rethink of how services should be reconfigured to enhance care for cancer survivors (Reference StovallStovall et al., 2006). The increasingly chronic nature of cancer means that survivors require ongoing support and care in specialist settings and the community. Guidelines for follow-up and survivorship care are being developed in many countries, and research and development into interventions and service development are on-going. Cancer and cancer treatments are associated with a wide range of physical and psychological challenges, some of which may even appear only years after the initial treatment. Person-centred and stepped care approaches are considered to be the most appropriate way forward, but evidence remains weak on the best ways of providing care that optimizes symptom treatment and problem solving for different cancer sites. For example, Reference TsianakasTsianakas et al. (2012), in a study of patient experience of cancer services, found that while those with breast and lung cancer reported broadly similar experiences, they differed in the nature of information they required and the priorities they attached to service improvement activities. New care models are emerging that emphasize the importance of supporting patients to engage in self-management activities and to enable them to make informed choices about the type of support they need. It is apparent that cancer survivors must become more effective coproducers in their own care, with Reference TsianakasTsianakas et al. (2012) proposing experience-based codesign as an approach to ensure that patients will be involved as active partners in the care process.
Patient empowerment will be at the core of new approaches to cancer care in hospitals, with Reference GroenGroen et al. (2015) identifying five key attributes: (1) being autonomous and respected; (2) having knowledge; (3) having psychosocial and behavioural skills; (4) perceiving support from community, family, and friends; and (5) perceiving oneself to be useful. The latter two are essential in the cancer setting. Information and communication technology (ICT) and eHealth initiatives are playing an increasing role in supporting cancer patients (Reference McAlpineMcAlpine et al., 2015). Technology ranges from electronic patient portals to electronic decision aids or online cognitive behavioural therapy programmes. The majority of eHealth initiatives in cancer care tends to focus on providing patients with information about their disease and treatments to enable shared decision-making, with only a minority aimed at patients to build the skills necessary to cope with the symptoms they experience (Reference GroenGroen et al., 2015).
The fragmentation of ICT services poses a major challenge to realizing their potential benefits. In the Netherlands, for example, many hospitals have their own patient portal system and do not easily connect to other systems in the cancer care trajectory. If they are to increase patient-centredness and shared care, IT services need to be linked in a way that makes information available for every health care provider in the chain (“shared care”). This most probably will lead to a medical record that is owned by the patient and has connections to multiple health care providers.
Organizational trends in cancer care and hospital-based oncology across the EU
Networks
The development of advanced diagnostics in nuclear medicine, MRI, molecular pathology, and genetic sequencing requires specialist staff and investments in infrastructure and equipment (see Chapter 9). In combination with a growing awareness that in some areas hospitals with a greater volume of patients with certain conditions achieve better outcomes, we see a gradual trend towards cooperation between hospitals in networks, with centralization, especially of complex low volume interventions. This requires providers to formalize agreements on cooperation, division of labour and handovers, and to discuss pathways across organizational boundaries. In 2018 the Organisation of European Cancer Institutes (OECI) started the development of Patient-centred Quality Standards for Cancer Networks (Organisation of European Cancer Institutes, 2018). We here use the examples of the Netherlands (Box 5.1) and Italy (Box 5.2) to illustrate these trends.
Box 5.1 The emergence of cancer networks in the Netherlands
In the Netherlands most hospitals provide cancer care, although there has been a steady trend towards the centralization of, in particular, low volume and complex treatments. University centres specialize in rare cancers and large hospitals have a leading role in the treatment of high volume tumours such as breast, lung, colorectal and prostate cancer. This trend was initially stimulated by the setting of minimum volume standards for various procedures by government and health insurance companies. This role has now been taken on by professional associations, which are defining norms and quality criteria; they have also introduced quality registries.
The Netherlands Comprehensive Cancer Organization (IKNL) is the quality institute for oncological and palliative research and practice in the Netherlands. It collaborates with health care professionals and managers and patients on the continuous improvement of oncological and palliative care, encourages knowledge exchange and organizes consultation service between centres of expertise and regional hospitals. IKNL also coordinates the issuing and maintenance of care guidelines (Volksgezondheid en zorg, 2016).
In response to the centralization of cancer services, and to clarify the role of various types of hospital and university medical centres (UMCs), regional cancer centre networks (CCNs) are being established. The CCNs will focus on improving treatment, care and clinical research in oncology across the network. It remains challenging to implement CCNs, however, as not all UMCs cover all relevant high volume tumours. Importantly, so far the Netherlands has only one comprehensive cancer centre – the Netherlands Cancer Institute – that has received formal accreditation by the OECI. Also, it was only recently that it was decided to concentrate all paediatric oncology in one national centre (construction started in 2016). As the total number of new cases amounts to around 500 per year, this guarantees state-of-the-art expertise for every individual case (Prinses Maxima Centrum, 2016).
Box 5.2 The emergence of cancer networks in Italy
The Italian National Cancer Plan is increasingly focusing on the concept of regional oncology networks, according to a federal model, of which the Lombard Oncology Network (Regione Reference LombardiaLombardia, 2006) is the most representative example in the national network Alleanza Contro il Cancro. Locally, the network provides significant benefits in terms of resources and information optimization; at a national and international level it helps to maximize the collaboration and the sharing of best practices.
The EU has established European reference networks (ERNs) for rare diseases, with networks for paediatric, haematological, and solid tumours approved in December 2016. These are intended to improve the quality of care, to coordinate knowledge dissemination and to facilitate cross-border care. By early 2017 the first European reference networks became operational (European Commission, 2018). Reference PalmPalm et al. (2013) highlighted various challenges in the implementation of European reference networks. For example, for Denmark it was shown to be challenging to identify the right national balance in terms of geographical coverage and capacity of “good clinics”, and for monitoring and evaluating the system. There remain questions about how the concept of reference centres and networks should be defined, the management of the process of identifying centres, and the implications of the establishment of such networks for funding of services and coverage of the population.
Organization
There is an increasing trend to centralize cancer services through the formation of cancer centres (outside or within the hospital structure), with a growing number of hospitals and cancer centres in a range of EU countries entering the Accreditation and Designation (A&D) programme of the OECI (Organisation of European Cancer Institutes, 2018).
There remains debate about the optimal model of organizing cancer care; the added value of different forms is difficult to establish, with little robust evidence on the best way of delivering cancer services.
Germany provides an example of a distinct organizational model, which involves a three-tier approach (DKG German Cancer Society, 2014). The first tier comprises comprehensive cancer centres (Onkologische Spitzenzentren), which are the leading oncology centres holding a major research portfolio. The cancer centres focus primarily on rare cancers and specialized aspects of care, with a specific programme by the Deutsche Krebshilfe periodically designating 14 centres as comprehensive cancer centres, which will receive considerable additional funding for their translational research. The second tier includes oncology centres, which cover several cancer sites or specialties, particularly rare cancers. The designation as an oncology centre is led by the German Cancer Society (GCS) and aims to guarantee high quality of services for payers, the public and government. The third tier includes organ cancer centres, which specialize in one organ or specialty (e.g. breast, bowel, lung, prostate, skin, and gynaecological tumours). The organ cancer centres are also covered by the GCS programme (DKG German Cancer Society, 2014).
At the European level there are various professional and institutional oncology societies. Professional societies include the European Society of Medical Oncologists (ESMO) and the European Society for Radiotherapy and Oncology (ESTRO). An example is the European Cancer Organization (ECCO), a multidisciplinary organization that connects all stakeholders in oncology across Europe. ECCO is a not-for-profit federation that aims to uphold the right of all European cancer patients to the best possible treatment and care, promoting interaction between all organizations involved in cancer at European level (ECCO, 2016). Another is the Organization of European Cancer Institutes (OECI). The OECI is a non-governmental, non-profit organization with the primary objective to improve communication and bringing together cancer research and care institutions across the European Union, in order to create a critical mass of expertise and competence (Organisation of European Cancer Institutes, 2016).
Patient registries
Patient registries (to be distinguished from the more traditional cancer registries) that collect and enable the monitoring of data on treatments and tumour characteristics have been established in Norway, Sweden and, more recently, the Netherlands. The population-based cancer registries in the Nordic countries include more comprehensive disease-specific quality registries covering treatment data and detailed outcomes data (Reference MøllerMøller et al., 2002).
In Italy the Italian Association of Cancer Registries (AIRTUM) established a network of registries which gained international importance in contributing to European survival studies (Reference AngelisDe Angelis et al., 2014), international prevalence comparisons (Reference CrocettiCrocetti et al., 2013) and the consolidation of partnerships within the network of cancer registries in the Mediterranean area, including the southern coast (Reference Hamdi CherifHamdi Cherif et al., 2015), through the Euromed (EEAS, 2016) project.
Cancer Care in the hospital of the mid-21st century
Cancer will account for a growing volume of hospital activity in coming years. Not only does this mean that more patients will be offered innovative and promising treatments, but the sustainability of the health system, in terms of human and financial resources, will remain under continuous strain.
Perhaps the most important message from this chapter is that the care of cancer in hospitals has become vastly more complex than in the past, in terms of both the technical ability to characterize and understand tumours and to individualize their treatment, and the organizational responses that bring together staff with differing types of expertise. This will require close coordination between clinicians and managers. Further concentration and specialization of services will be inevitable, and this will require continuing networking, increasingly using innovative information technology, telehealth, and telemedicine to ensure that concentration of services does not undermine geographical access to services. It is very likely that regional networks of hospitals will organize cancer care among themselves, concentrate and share expensive diagnostics and intervention capacity, and establish liaisons with referral centres of expertise for rare tumours. Networks of reference centres are established in the EU and offer considerable potential for promoting research and innovative treatments for patients with some very rare tumours, or tumour subtypes.





