Child health care in Europe
The role of the hospital in caring for children has changed beyond recognition in the past five decades. On the one hand, the conditions that were once responsible for most bed occupancy, such as respiratory tract infections, gastroenteritis, and hepatitis A, are now far less common and, when they occur, are managed at home in all but the most severe cases. On the other hand, advances in medicine and technology, coupled with better understanding of genetics, metabolic and neonatal medicine, new treatments for cancer and acute/chronic organ failure, advances in surgical techniques, and new ways of managing severe mental disorders, have created a need for services that did not previously exist (Reference WolfeWolfe et al., 2013). Consequently, the hospital continues to play a key role in the health care of children, albeit one that is rapidly adapting to the changing needs of sick foetuses, newborns, infants, children, and adolescents. Hospital services for children must also be able to work closely with other parts of the health system and beyond, reaching out to wider services for children including education, prevention, long-term outpatient care for children with rare diseases, and primary care out of normal hours. Yet a survey conducted in 2015 revealed great diversity in hospital services for children in the 53 countries of the World Health Organization’s European region (Reference Ehrich, Namazova-Baranova and Pettoello-MantovaniEhrich, Namazova-Baranova & Pettoello-Mantovani, 2016). Differences are apparent even at the most basic level: the definition of a child. The age at which young people are no longer managed in children’s hospital services varies among countries. In 53% of countries childhood is defined as up to 18 years of age, but in one country it is up to 11, in three up to 14, in four up to 15, in six up to 16, and in one up to 17 years of age. Two countries reported the upper age limit for children in paediatric services to be 19 and in one country it is 26 years (Reference EhrichEhrich et al., 2015a).
There are also considerable variations in the settings in which children receive hospital care. A 2009 survey conducted by the European Paediatric Association identified four different types of children’s hospital in Europe: 1) general children’s hospitals and paediatric units (or paediatric wards) within larger hospitals for adults; 2) stand-alone children’s hospitals; 3) university children’s hospitals; and 4) mother and child centres. Day clinics and neonatal intensive care units (NICUs) were found in all four types of hospital. There are also major differences in infrastructure, such as diagnostics and therapeutics, especially high technology equipment, as well as organizational arrangements and markers of quality.
As with every other aspect of medicine, health services for children must adapt to a rapidly changing landscape. One way in which this landscape is changing is the demography of Europe. With a falling birth rate, Europe is facing a declining child population. The mean shares of the total population aged 0–14 years and 0–4 years in Europe were 21.2% and 6.4% respectively in 1982 but these figures had fallen to 15.2% and 5.0% in 2014. The scale of the change can be seen from looking at three countries, Belgium, Ireland, and Portugal, where the fertility rate decreased from 2.54, 3.78 and 3.16 respectively in 1960 to 1.75, 1.96 and 1.21 in 2013 respectively (Eurostat, 2016).
This demographic change, coupled with changes in disease patterns and treatment settings, has contributed to a large reduction of hospital beds and to the closure or merging of children’s hospital facilities and thus is challenging conventional ways of thinking about hospital facilities for children. Traditionally based on a division between primary, secondary, and tertiary care, new models of care seek ways to innovate and improve the whole system. The changes are complex and do not simply involve crude reductions in hospital capacity. For example, on the one hand, the decline in the incidence of communicable diseases through immunization programmes, as well as injuries through injury prevention programmes, has caused a decrease in the need for care and consequently hospital admissions. On the other hand, medical and surgical advances, in areas such as neonatal surgery and intensive care, oncology, and interventions for inherited diseases, are increasing the need for highly specialized care that can only be provided in tertiary hospitals. At the same time, there is a continuing burden of chronic diseases – some attributable to increasing risk factors, such as childhood obesity, and some to improved survival of previously fatal conditions, such as malignancies and certain inherited disabilities. This has created a greater need for specialist care that transcends the hospital and the community (Reference Wolfe and McKeeWolfe & McKee, 2014), a need that is also increasing because of the improved survival of very low birthweight babies, some of whom are living with long-term disabilities. These children require sophisticated diagnostic and therapeutic interventions delivered by well-trained personnel using technologically advanced infrastructure.
In addition to the complications created by the increasing specialization of care provided for children in hospitals, there is a growing recognition of the importance of designing systems from the perspective of the user rather than the provider of services. This is exemplified by the call to design “a hospital that does not feel like a hospital”. This thinking has been captured in the Council of Europe’s “Child-Friendly Health Care” approach, which was endorsed by 47 ministers representing the nations of Europe (Reference Lenton and EhrichLenton & Ehrich, 2015) (Box 2.1). This approach brought systems thinking and values based on the United Nations Convention on the Rights of the Child together into a practical framework to plan, deliver and improve services for children and families. The child-friendly health care approach builds on patient-centred care and patient pathways. The responsibility of the health system is to ensure that all the component parts are in place and working well together to achieve the best possible outcomes. This can only happen if appropriate child health care networks, based on collaboration (Future Ho . This spital Commission, 2013), can work together to improve quality continuously. Crucially, the hospital is a key element of these networks. Despite the shift from inpatient to outpatient care and the fall in the mean duration of stay in hospital the hospital is still very important. Although their work often extends beyond the walls of the hospital into the community, it is still the case that in many European countries about half of all paediatricians are still hospital-based (Reference EhrichEhrich et al., 2015a).
Box 2.1 Extract from the terms of reference of the Council of Europe on child-friendly health care
Five principles are particularly relevant to the child-friendly health care approach:
1. Participation Participation means that children have the right to be informed, consulted, and heard, to give their opinions independently from their parents, and to have their opinions considered. It implies the recognition of children as active stakeholders and describes the process by which they take part in decision-making. The level of child participation depends both on his or her age, evolving capacities, maturity, and on the importance of the decision to be taken.
Parents and families should encourage children to participate in family, community and society decision-making – encouraging increasing independence and reducing their support as the child’s capacity for autonomy and independence develops.
2. Promotion Health promotion is “the process of enabling people to increase control over their health and its determinants and thereby improve their health”. Promotion therefore includes all actions that allow
children to become more involved in their own health and increase their exposure to positive determinants of health (defined as factors which will improve health or well-being). Health promotion covers not only activities in families and communities, directed at health determinants or lifestyles, but also factors in health care services and settings which will improve outcomes.
3. Protection Health protection includes all actions that either limit or avoid children’s exposure to any hazard which can be defined as a factor that has the potential to cause harm. Hazards can occur in families, communities and health services. Medical interventions can cause harm and patient safety perspectives highlight the fact that children are particularly vulnerable to medication errors and hospital-acquired infections.
4. Prevention Prevention is an active process the aim of which is to avoid future health, social or emotional problems to enable the fullest realisation of human potential. This includes action to reduce adverse health determinants, to prevent the development of a disease or condition, to avoid complications of a disease or condition, to prevent the impact of a disease or condition on the lifestyle or aspirations of an individual, and to prevent harm caused by a service or intervention.
5. Provision Provision refers to any service which contributes to the health and well-being of children and families, and therefore includes more than just traditional health services. “Pathway-based provision” is a concept that describes all the component parts that need to be in place and working well together to achieve an excellent patient experience which brings about optimal outcomes for children and families in their journey through services.
Clearly, in the light of the preceding discussion, there is no simple way to structure a chapter on the care of children in hospital. Consequently, we have taken a pragmatic approach, looking first at the highly-specialized care of newborn infants, followed by the care of older children with common conditions requiring hospitalization, and then the provision of highly specialized care in tertiary hospitals. We then review some common patient pathways, illustrating the inter-linkages between the different elements of the system before looking to emerging developments that may impact on the health system response to children in the future.
Maternal, neonatal and follow-up care in specialized facilities
Trends in obstetric and neonatal care
The management of pregnancy and childbirth has been transformed in recent decades, both organizationally and in terms of the technology and knowledge base required to achieve improved outcomes. These changes have been accompanied by a marked improvement in outcomes. Neonatal mortality has improved substantially during the last four decades. In 1975 the 28 current European Union Members (EU28) experienced 12.84 neonatal deaths per 1000 live births, yet by 2014 this had fallen to 2.52 per 1000 live births. This reflects several factors, including a reduction in low birthweight babies, and especially, survival among those born prematurely. Consequently, in 1975 half of all premature newborns with a birthweight less than 1500 g died during the postnatal period but this fell to 14.3% and 12.4%, in 2000 and 2009 respectively (European Society for Neonatology, 2015). Maternal morbidity and mortality have also improved significantly.
These changes have been accompanied by several, often conflicting, trends taking place in the organization of services during childbirth in European countries that have implications for the future role of the hospital. In the past, many deliveries took place in small local hospitals, close to where people lived. These hospitals often had limited facilities but had the advantage of convenience for the mother and her family. However, the falling birth rate in many countries and the subsequent reduction in demand for delivery facilities threatens the viability of these hospitals, many of which have closed for other reasons, such as the inability to provide comprehensive, 24-hour, advanced medical and surgical services. Moreover, the smaller hospitals were unable to provide the facilities required when complications arose during childbirth. Yet, while many of the other services that these hospitals once provided have been transferred to larger hospitals with more sophisticated equipment, there is also pressure to de-medicalize childbirth, leading either to increased home births or to the development of stand-alone family friendly facilities, separate from acute hospitals.
Such facilities clearly meet the needs of the majority of expectant mothers. However, there are some who have other conditions that place them at high risk, such as advanced cystic fibrosis or cardiac insufficiency, or who are post-transplant, who require careful monitoring by a MDT that brings together adult medical, obstetric, and neonatal care. In the past, many of these mothers would not have survived into adulthood, and those who did would have been advised against becoming pregnant. With individualized care planning for delivery, coupled with advances in intra-partum care, they can now expect to have a healthy baby. However, this intensity of management, and especially the involvement of multidisciplinary teams, can only be undertaken from a well staffed and equipped hospital facility, even though those expectant mothers that do require specialized medical or surgical intervention can often receive it on an ambulatory basis. An added complication is that in some countries there have been increases in the number of infants who require intensive care and specialist intervention, in part reflecting later pregnancies and multiple births following in vitro fertilization. Finally, as often noted, a normal delivery can only be assessed as such in retrospect.
For these reasons, there is a need to ensure close coordination between facilities undertaking deliveries, whether stand-alone or within acute general hospitals, and those facilities providing specialized neonatal care. Ideally, any pregnancy identified as high-risk should be delivered in a setting where the delivery suite and the NICU are adjacent, or at least on the same site, but it is also important to recognize that, while unanticipated complications of delivery are fortunately rare, they do happen, so there should also be mechanisms in place to enable early referral and rapid intervention to save the life of the mother and baby in stand-alone facilities.
There are other reasons for close collaboration between obstetric and neonatal services, including shared training and participation in research, especially that responding to the needs of mothers and babies with complications. Obstetrician involvement in postnatal NICU ward rounds and discussions with parents can improve the knowledge base for antenatal counselling, which should also ideally involve the MDT, including the obstetrician, neonatologist, and, where appropriate, teams providing surgical and highly specialized paediatric expertise.
This is, however, an area where technology and knowledge continue to advance rapidly. New resuscitation guidelines have recently been published by ILCOR/ERC/AHA in 2015 (Reference WyllieWyllie et al., 2015), including changes in resuscitation practice, such as resuscitation closer to the mother to allow delayed cord clamping, but requiring greater involvement of specialist neonatal care in the delivery suite. At the same time, advances in remote monitoring are making it possible for senior staff to provide input remotely during resuscitation. Other advances include greater use of point-of-care testing (POCT), discussed further in Chapter 10, but this will require adaptation, including the use of nanotechnology, to take account of the very small volumes of blood that can be taken from extremely premature infants.
Provision of NICU
Existing guidance suggests that in a typical western European country, based on contemporary practice, there is a need for 0.75 cots per 1000 births for intensive care, 0.75 cots per 1000 births for high dependency care, and 4.4 cots per 1000 births for special care (Reference LaingLaing et al., 2004). However, this must also take account of changes in the frequency of preterm births, such as the increases in several countries including the USA, Canada, Australia, Sweden, Scotland, and Wales (Reference HallsworthHallsworth et al., 2008). In Europe, in countries with comparable levels of development and health care systems, preterm birth rates vary markedly, ranging from 5% to 10% among live births. A second question relates to the distribution of facilities. A German population-based study found that 28-day mortality was more closely associated with the numbers of neonates looked after in a NICU than with the number of births in the hospital, with the effect greatest for infants of less than 29 weeks’ gestation (Reference HellerHeller et al., 2007), although a study in the USA found that, while both the number of very low birthweight babies and the numbers treated in NICUs were important determinants of good outcomes, the former was more important. Other researchers found that mortality in small NICUs is significantly increased (Reference BartelsBartels et al., 2006). This evidence has led the American Academy of Pediatrics, in its Committee on Fetus and Newborn report on Levels of Neonatal Care, to support larger-volume NICUs.
NICU design and environment
As survival of preterm infants has improved dramatically during recent decades, there has been a marked increase in the number of children treated in NICUs. Initially, in the late 1970s and 1980s, NICUs were designed as multipatient wards with some private rooms to isolate infants with infections. In the 1990s, as survival became commonplace, new ideas began to emerge about possible effects of the physical environment on the fragile, growing brain of newborns. In 1992 Reference White and WhitmanWhite and Whitman (1992) recommended some private rooms for neonates. Many studies have now found that preterm infants are influenced by the physical conditions in NICUs, such as noise (Reference Long, Lucey and PhilipLong, Lucey & Philip, 1980) and lighting (Reference MannMann et al., 1986). Box 2.2 sets out suggested environmental and building standards.
Box 2.2 Environmental and building standards for NICUs
Noise: Sound levels should be kept at less than 40 dB. Private rooms provide a decrease in the number of adults in the room, and a study by Reference Robertson, Cooper-Peel and VosRobertson, Cooper-Peel & Vos (1999) showed clearly that decreasing conversation had the greatest effect on decreasing noise levels in a NICU.
Light: Adjustable lighting between 0.5 and 60 ft-candles (5–600 lux) is appropriate for general lighting levels in NICUs and an indirect room lightening should be preferred. A circadian lighting scheme should be used in the patient care area.
Air quality: NICUs should be air-conditioned to the highest standards, with air temperature at 22–26 degrees Celsius, 30–60% relative humidity, and a minimum of six air-changes per hour.
Design: Careful design is needed, with extensive additional space for family, overnight stays, privacy and staff.
Private rooms: Single-family rooms (private rooms) allow infants to be cared for in a room where they are shielded from medical or social activity at a neighbouring bed. The risk of cross-contamination may also be reduced.
The first all-private room NICU in Europe was built in Brest, France, for the express purpose of minimizing nosocomial infection (Reference WhiteWhite, 2011), although no study has yet found that private rooms in NICUs enhance infection control. A study from the Karolinska group of hospitals in Stockholm compared the results of two different types of NICU: those with private rooms versus those with four-bed open rooms (OReference Ortenstrandrtenstrand et al., 2010). Premature newborns cared for in private rooms showed marked reduction in ICU and total hospital days, as well as a reduction of bronchopulmonary dysplasia. However, recent research has highlighted the need for greater attention to the sound in the NICU as infants nursed in single rooms had significantly altered MRI findings compared to those in an open ward (Reference SmithSmith et al., 2011).
Guidelines developed within the WHO/UNICEF Baby-friendly Hospital Initiative (BFHI) (World Health Organization, 2016) propose that newborns who do not need NICU facilities should be cared for in their mother’s room, with the support of specialized nurses to encourage and support bonding and support breastfeeding. Family rooms that allow parents to “room-in” and care for their infants also offer a means for siblings to meet and bond with the new baby without creating infection-control issues for the NICU.
Levels of newborn care
Newborns need different levels of care in NICUs. The 2012 classification developed by the American Academy of Paediatrics is used widely (American Academy of Pediatrics, 2012). These facilities are divided among those providing basic care (level I), specialty care (level II), and subspecialty intensive care (level III, level IV). Level I facilities (well newborn nurseries) provide a basic level of care to neonates who are low risk. Neonatal resuscitation can be undertaken if required for every delivery in these units and healthy newborns can be evaluated and receive routine postnatal care. In addition, Level I units can care for preterm infants at 35 to 37 weeks’ gestation who are physiologically stable, and can stabilize newborn infants who are less than 35 weeks’ gestation or who are ill until they can be transferred to a facility at which specialty neonatal care is provided. Care is provided by paediatricians, family physicians, and nurse practitioners. The recommended ratio of nurses to babies is 1:4. Interestingly, a study of NICUs in California found no difference in quality across levels of NICU (Reference ProfitProfit et al., 2016).
The British Association of Perinatal Medicine (BAPM) has published guidelines suggesting that units with fewer than 50 infants with birthweight less than 1500 g should plan to amalgamate with other units to ensure clinical skills and expertise are retained (British Association of Perinatal Medicine, 2010). The BAPM guidelines also highlight the importance of hand-washing facilities for parents and staff, as well as adequate space to prevent cross-infection between babies and isolation facilities for infected infants.
Care in a specialty-level facility (level II) should be reserved for stable or moderately ill newborn infants who are born at ≥32 weeks’ gestation or who weigh ≥1500 g at birth but have problems that are expected to resolve rapidly and who would not be anticipated to need subspecialty-level services on an urgent basis. Level II nurseries may provide assisted ventilation until the infant’s condition either soon improves or the infant can be transferred to a higher-level facility. Care is provided by neonatologist and neonatal nurse practitioners (NNPs) in addition to level I staff. The recommended ratio of nurses to babies is 1:2.
Infants who are born at less than 32 weeks’ gestation, weigh less than 1500 g at birth, or have major medical or surgical conditions, regardless of gestational age, should be cared for at a level III facility. Level III facilities should be able to provide ongoing assisted ventilation for 24 hours or more, which may include conventional ventilation, high-frequency ventilation, and inhaled nitric oxide. A broad range of paediatric medical subspecialists and paediatric surgical specialists should be readily accessible on site or by prearranged agreements. Level III facilities should have the capability to perform advanced imaging with interpretation on an urgent basis, including CT, MRI, and echocardiography. Care is provided by paediatric medical subspecialists, paediatric anaesthesiologists, paediatric surgeons, and paediatric ophthalmologists in addition to level II staff. The recommended ratio of nurses to babies is 1:1.
Level IV units include everything that is available at level III with additional ability to care for the most complex and critically ill newborn infants. Such units should have specialist paediatric medical and surgical consultants continuously available 24 hours a day. Level IV facilities also include the capability for surgical repair of complex conditions (such as congenital cardiac malformations that require cardiopulmonary by pass with or without extracorporeal membrane oxygenation). Care is provided by level III staff plus paediatric surgical subspecialists.
Good outcomes for neonates in the NICU are dependent on the availability of sufficient numbers of skilled neonatal nurses. Developing NNPs can help maintain skills and continuity of care as medical staff change frequently. In many countries NNPs are expanding into roles such as neonatal transport and NNP-led clinics. Clinical nurse specialists provide vital services in the areas of discharge planning, support for lactation, and resuscitation. In some areas community neonatal nurses provide pre- and post-discharge care and visits. Clinical nurses and midwives specializing in bereavement play an important and expanding role in maternity hospitals, supporting parents faced with an antenatal diagnosis of a potentially lethal condition or who have newborns who die in the first few weeks of life. The United Kingdom’s Royal College of Paediatrics and Child Health (RCPCH) framework on withholding or withdrawing life-sustaining treatment in children and the neonatal palliative care guidelines offer guidance to health care providers in these situations. These health professionals can also address issues such as neonatal organ donation.
Speech and language therapists, dieticians, physiotherapists, psychologists, medical social workers and occupational therapists are all essential to the operation of a level III NICU. Neonatal dieticians are playing an increasing role in optimizing nutrition. The extended role pharmacist will become more important in contributing to staff and parental education on medication use and prevention of prescribing errors, as well as new pharmaceutical agent use in the NICU. The role of clinical engineers has expanded with newer devices with a broader range of uses, such as neonatal ventilators and monitoring equipment for transport and care of infants with neuro-critical conditions. Neonatal transport is an essential part of an integrated neonatal network. This service needs to be available round-the-clock, including specialized equipment such as that required for hypothermia therapy.
Beyond the classification of NICUs set out above, there is a need to consider separately the provision of extracorporeal membrane oxygenation (ECMO), a life support mechanism which allows blood to be taken from the body, oxygenated outside the body and returned, and carbon dioxide and oxygen exchanged. A randomized controlled trial conducted in the United Kingdom showed a clear benefit for newborn infants with severe respiratory failure.
Education for staff and families
The European Society of Neonatology (ESN) Curriculum for Training in Neonatology in Europe (European Society for Neonatology, 2015) was developed to support national training programmes. The ESN has created a database of national training programmes to encourage transparency and harmonization of subspecialist training in neonatology.
Technological advances have enabled simulation to become a core element of training, with dedicated simulation laboratories equipped with high-fidelity mannequins in many new level III units. This development has been encouraged by the implementation of the European Union (EU) working time directive, designed to reduce the known risks associated with long working hours. However, despite the clear benefits for patient safety, it has posed problems in enabling medical trainees to obtain adequate practical experience. Simulation offers a means to deliver carefully designed, well supervised training experiences that are a significant improvement over the ad hoc approaches used previously. Simulation also offers a means to provide coordinated training for the multidisciplinary teams whose work is now so essential in NICUs, allowing them to develop their skills in a team setting where they can realistically model clinical scenarios.
Reflecting the important role that parents play in the care of newborn infants, it is important that training should not be limited to staff. Although parents are supported as they come to terms with the health of their newborn infants, there is considerable scope to develop this more formally in association with NICUs, including preparation for those expectant parents where it is anticipated that their babies are likely to require a stay in a NICU, as well as preparation for the post-discharge period.
Secondary care for children
While much childhood illness can be managed in primary care, there will inevitably be children who require hospitalization in secondary care facilities. Table 2.1 sets out some examples of such conditions. However, the numbers involved will depend not only on the burden of disease in the population but also on the scope and quality of primary care, which varies greatly around Europe. Policies in many countries have sought to reduce unnecessary admissions to hospital as they are
Table 2.1 Selected examples of indications for admission of children to hospitals
| Paediatric subspecialty care | Standard indications | Optional* indications |
|---|---|---|
| Neurology | developmental disorders, di-/tetraplegia, gait disorders, headache, neuropathies, seizures, etc. | myopathies, motor, hearing, visual, mental and skeletal disabilities, specific sleep disorders, etc. |
| Ear, nose and throat | tonsillitis, otitis, sinusitis, lymphadenopathies, etc. | cholesteatoma |
| Cardiology | arterial hypertension, arrhythmias, myocarditis | cardiomyopathy |
| Pulmonology | laryngitis, bronchi(oli)tis, asthma, pneumonia | cystic fibrosis |
| Hepato-gastroenterology-Nutrition | gastroenteritis, appendicitis, hepatopathies, abdominal pain, etc. | chronic inflammatory bowel disease, intussusception, cholestasis, etc. |
| Hematology/Hemostaseology | anaemia(s), leukopaenia, immune thrombocytopaenia (ITP), preoperative screening | coagulopathies |
| Infectious diseases | meningitis, encephalitis, upper airway infections, hepatitis, borreliosis, etc. | tuberculosis |
| Urology/Nephrology | urinary tract infection, hydronephrosis, glomerulonephritis, etc. | common nephritic and nephrotic syndrome |
| Dermatology | all kinds of rashes, atopic dermatitis | haemangioma |
| Mental disorders | somatoform disorders | ADHD, depression |
distressing for children, cause problems for parents, and are usually a less cost-effective way of treating acute illness. On the other hand, delayed referral of severely sick children to hospital may lead to preventable complications and death. Closing the organizational gaps between primary and secondary care for children is therefore an important task.
It is important to see the hospital as only one element within the wider health system. However, the way in which the hospital interacts with the other elements of the health system will vary, influenced by the organizational characteristics of the system. Across Europe, the responsibilities of hospitals caring for children are not uniformly defined and vary between countries and even regions within countries. Furthermore, they may vary according to whether the hospital is publicly or privately owned, with the former typically responsible for providing a comprehensive range of services while the latter can select those areas that are most profitable and incur least risk to the provider. Figure 2.1 illustrates these relationships, with the hospital bringing together a range of specialist expertise.

Figure 2.1 The position of hospital care for children within the health system
Where in the system a child is treated will vary according to a range of factors. However, even within a single system, the boundaries are not necessarily clear. Decision-making processes relating to treatment and referral are subject to different rules and regulations of the health systems, but also policies about what services to offer in what facility, themselves influenced by the interests of the health care personnel involved. For instance, the management of many long-term conditions, such as asthma, diabetes, or coagulopathies, may be provided in different settings in different countries. Despite the existence of such variations, it is desirable that those responsible for managing and providing care in hospitals should find ways to achieve consensus with primary care physicians and tertiary care paediatricians on standards to be adopted for infrastructure, facilities, staff, and quality of medical treatment.
Recognizing that, where possible, children and adolescents should be managed in settings other than in hospitals, when they are admitted they are entitled to have certain expectations:
To be welcomed by friendly staff, whether doctors, nurses or others
To be adequately informed about what is to be done and when;
To have the option to be accompanied by mother, father, and other relatives;
To be treated according to modern standards of evidence-based medicine;
To experience as little pain as possible;
To receive age- and disease-appropriate treatment;
To be treated by staff trained to communicate with children and parents;
To experience a private and respectful atmosphere whenever possible;
To be properly informed about all procedures and results of investigations;
To stay in hospital for as short a time as possible;
To receive adequate information about further treatment;
To receive full treatment free of charge.
These expectations of patients and their care takers are consistent with the Charter of the European Association for Children in Hospital (EACH) (Box 2.3) (European Association for Children in Hospital, 2015).
Box 2.3 Charter of the European Association for Children in Hospital (EACH)
Article 1 Children shall be admitted to hospital only if the care they require cannot be equally well provided at home or on a day basis.
Article 2 Children in hospital shall have the right to have their parents or parent substitute with them at all times.
Article 3 Accommodation should be offered to all parents and they should be helped and encouraged to stay. Parents should not need to incur additional costs or suffer loss of income. In order to share in the care of their child, parents should be kept informed about ward routine and their active participation encouraged.
Article 4 Children and parents shall have the right to be informed in a manner appropriate to age and understanding. Steps should be taken to mitigate physical and emotional stress.
Article 5 Children and parents have the right to informed participation in all decisions involving their health care. Every child shall be protected from unnecessary medical treatment and investigation.
Article 6 Children shall be cared for together with children who have the same developmental needs and shall not be admitted to adult wards. There should be no age restrictions for visitors to children in hospital.
Article 7 Children shall have full opportunity for play, recreation and education suited to their age and condition and shall be in an environment designed, furnished, staffed and equipped to meet their needs.
Article 8 Children shall be cared for by staff whose training and skills enable them to respond to the physical, emotional and developmental needs of children and families.
Article 9 Continuity of care should be ensured by the team caring for children.
Article 10 Children shall be treated with tact and understanding and their privacy shall be respected at all times.
Highly specialized paediatric centres (tertiary care)
Expert specialist care is essential for the diagnosis and treatment of rare conditions and for children who require complex investigations and highly technical interventions, such as transplantation. This care typically requires sustained collaboration between different specialists and subspecialists to ensure optimal outcomes. However, while anecdotally it is known that there are different models of care, these have not, to our knowledge, been documented in detail.
Less well resourced countries in central and eastern Europe face the dilemma of how best to develop and fund specialist care in the future. Better resourced countries in western Europe face the problem of how best to rationalize and co-locate interdependent specialist services to improve outcomes. Small countries must find ways of developing effective cross-border care with larger countries, drawing on the many existing examples such as that between Malta and the United Kingdom (Reference SalibaSaliba et al., 2014).
One of the key questions facing those organizing specialized paediatric services is how best to balance centralization and decentralization. There are various arguments for creating a small number of large centres that can concentrate expertise and equipment, can create multidisciplinary teams, and can provide 24-hour services where necessary. The last of these is particularly important as it typically requires about 10 individuals to provide round-the-clock service, a number that can only be justified if there is sufficient caseload. The question of whether concentration of services achieves better outcomes has been debated extensively. There is clear evidence to support this for some services, such as neonatal intensive care and cardiac surgery. However, the evidence is rather more limited in other areas. It is also important to recognize that concentration of services in large centres, especially in countries with low population densities, can create a significant barrier to access, although it may be possible to compensate for this by the development of outreach services, whereby specialists travel from tertiary centres to other facilities. These decisions about how to provide highly specialized services are complex and require many, often competing, objectives to be balanced (Reference EhrichEhrich et al., 2015a).
These decisions must also be informed by considerations of which specialties should be co-located. The current situation is characterized by significant differences in care across European countries. One fact is the absence of consistent European definitions of either specialist care or specialist centres. There are also differences in training programmes and assessment, both within and between specialties, with 38 different accredited paediatric subspecialties reported in a European Paediatric Association survey in 2014 (Table 2.2), which exceeded the 22 recognized in the USA in 2012. Individual European countries recognize between 0 and 20 separate paediatric subspecialties (Reference EhrichEhrich et al., 2015a). This also poses challenges to those organizing training programmes, especially where the numbers of physicians in particular subspecialties are very low. However, the situation is further complicated by the scarcity of data on the numbers and qualifications of specialists in most countries. There is also little information on scope of practice and required competencies.
Table 2.2 Paediatric subspecialties in child health and the number of European countries in which each is recognized
| Adolescent medicine | 1 | Neonatology | 16 |
| Allergology | 8 | Nephrology | 12 |
| Anaesthesiology | 2 | Neurology | 14 |
| Cardiology | 14 | Neuro disability | 1 |
| Community paediatrics | 1 | Neuropsychiatry | 5 |
| Dermatology | 2 | Oncology | 12 |
| Developmental paediatrics | 1 | Ophthalmology | 3 |
| Emergency paediatrics | 5 | Orthopaedics | 2 |
| Endocrinology | 13 | Otorhinolaryngology | 3 |
| Gastroenterology | 13 | Palliative paediatrics | 1 |
| Genetics | 2 | Pharmacology | 1 |
| Gynaecology | 2 | Pneumonology | 12 |
| Haematology | 8 | Primary care paediatrics | 5 |
| Hepatology | 2 | Radiology | 3 |
| Immunology | 3 | Rehabilitation | 3 |
| Infectious diseases | 4 | Rheumatology | 8 |
| Intensive care | 9 | Stomatology | 2 |
| Mental health | 1 | Surgery | 6 |
| Metabolic diseases | 5 | Urology | 5 |
Note: Those in bold are also recognized by the American Council of Pediatric Subspecialties in Pediatrics 2012.
While recognizing these difficulties, it is possible to suggest the most important interdependencies among specialties (Figure 2.2). Where possible, those services with clear interdependency should be co-located. For example, a centre undertaking organ transplantation should also have the expertise necessary to provide care in haemato-oncology, cardiology, nephrology, metabolic medicine, paediatric surgery, and a paediatric intensive care unit (grey squares), while subspecialties such as endocrinology (white squares) are not required. Tertiary care children’s hospitals should have departments of child psychiatry and psychosomatic care in the same building. Transition and transfer of adolescents from paediatric to adult care should also take place in the same hospital, where possible (Reference CrowleyCrowley et al., 2011). Finally, the teams caring for children in hospital include teachers, who face particular challenges in meeting the needs of children who may divide their time between hospital and home over a prolonged period.
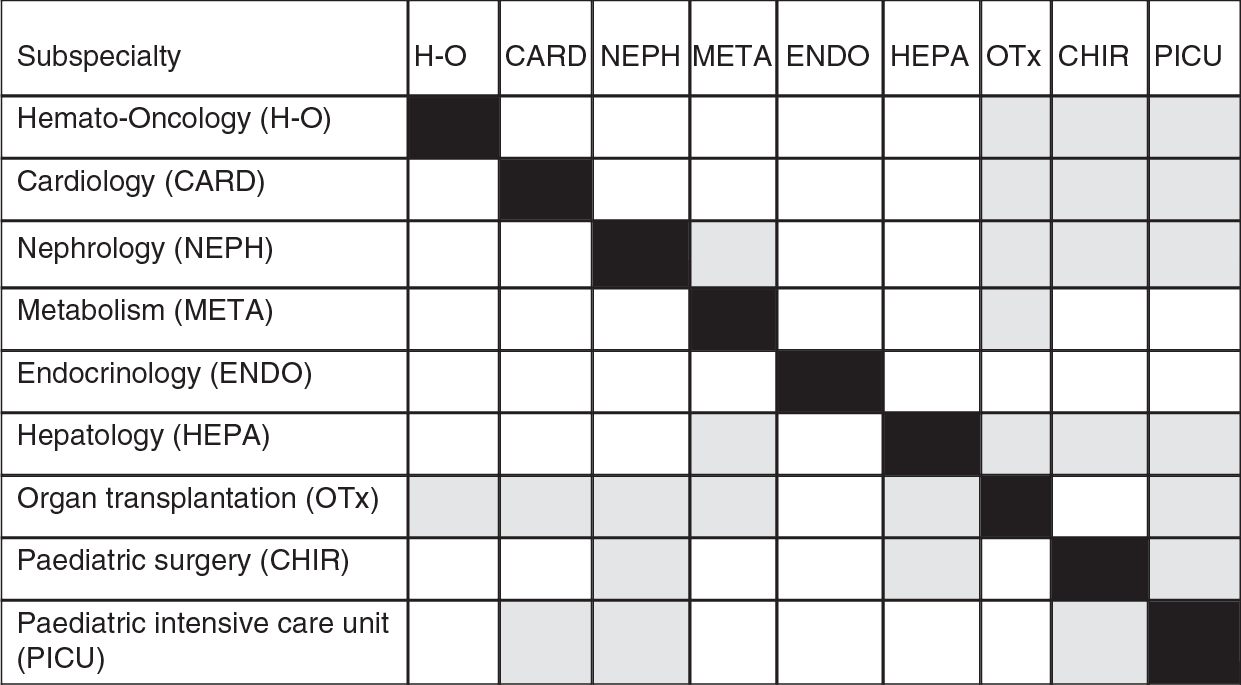
Figure 2.2 Appropriate co-location of paediatric subspecialties
The organization of services should be viewed from the perspective of both the child and their family and the health professionals working in the service. For the child and their family, it is important that all parts of the system should be in place and working well together with specialist advice easily accessible, but delivery should be as close to home as is safe and sustainable. This suggests the need to develop networked solutions where all those involved actively collaborate and constantly strive to improve safety and experienced outcomes. From the perspective
of the health professional, the specialist centre should not be seen as a “stand-alone” institution but as part of a well managed clinical network that promptly refers the most appropriate children and simultaneously receives children back into the local system for rehabilitation after specialist care. Clinical leadership for specialist care resides with the centre which organizes shared care with clear clinical care plans, with training and joint clinics for local teams. The local team should organize routine health and social care and education as appropriate. This model, based on good two-way communication, has already been achieved in some cancer and neonatal networks. The ideal system can be summarized with the phrase “centralized specialization and decision-making, but decentralized provision of treatment whenever possible”.
It is important to recognize that there is a risk that highly specialized paediatric subspecialty care may lead to fragmentation (Reference EhrichEhrich et al., 2015b). Consequently, especially where a child has multiple health problems, there is a strong argument for oversight of their care being undertaken by a general paediatrician, who can work closely with the child and their family. As Reference VohraVohra et al. (2012) state, “paediatric integrative medicine should be the paediatricians’ new subspecialty” to bring specialist care together.
In summary, there are many challenges in providing highly specialized paediatric care in Europe but there is a limited evidence base to inform the decision-making process. The most urgent questions needing answers include:
how best to plan an adequate number of specialist centres, where appropriate, taking account of possibilities created by the European Union Directive on Cross Border Care, so as to avoid both under-provision and over-supply;
how to develop a sustainable workforce to meet the medical needs of children; many different factors must be taken into account, including geography, population distribution, transport links, and relationships between centres;
how to balance any benefits from centralization with problems of access, recognizing that while most families will accept travelling long distances to receive episodes of specialist investigations or treatment, it is desirable that regular visits, for example for administration of treatment or follow-up, should take place as close to their homes as possible.
Typical patient pathways
We now look at the health system as seen through the eyes of the child. We do this by describing a series of journeys undertaken by children with four common conditions: an acute infectious disease, a chronic illness, a critical condition, and an illness requiring new technologies.
A child with acute infectious disease: acute lower respiratory tract infection (LRTI)
Acute LRTI in children – bronchiolitis and pneumonia – are normally managed in primary care by the first contact care giver. Should treatment fail, or in cases of atypical or recurrent pneumonias, the infant or child is referred to a secondary paediatric care setting (either ambulatory or inpatient facility) for further investigations, parenteral antibiotic therapy, oxygen and supportive treatment. Occasionally, a child with an LRTI might be found to have an underlying disorder, such as cystic fibrosis. In such cases, the child will be referred to a specialist team, normally at a tertiary care facility. The clinician treating the sick child must answer two questions. Is the infection due to a virus or bacteria? Antibiotic treatment is only indicated for the latter and unnecessary prescriptions increase the risk of antimicrobial resistance. Second, how likely is the child to respond to treatment, recognizing that small children in particular can deteriorate rapidly. On the one hand, it is necessary to ensure that further action is taken if there is such a deterioration. On the other hand, unnecessary admission to hospital should be avoided as far as possible. Advances in technology do, however, offer a possible solution as instruments based on evaluating gene expression profiles of leukocytes have demonstrated the ability to differentiate viral from bacterial infections, and scoring systems based on whole gene expression analyses may offer scope to assess severity in children with LRTI (Reference Wallihan and RamiloWallihan & Ramilo, 2014). In the future, these novel strategies may be able to identify rapidly those children who need antibiotics and those who should be promptly hospitalized.
A child with a chronic illness: asthma
If the asthma is moderate or severe, or if the diagnosis is uncertain, the child/adolescent is referred to a competent paediatric team either in an ambulatory paediatric setting or at the ambulatory clinic in a hospital paediatric department. Current recommendations are that such children should be referred to a specialist team at a regional centre if there are uncertainties about the diagnosis, or when children do not respond to recommended treatments. The paediatric teams should consist of specialized paediatricians and paediatric nurses. These teams will have access to lung function tests, blood tests and allergy testing. Ideally, the child will have a personalized treatment plan that is clearly documented, linked to a written asthma home management plan that is reviewed at every visit. Continued monitoring of asthma therapy is essential. Some centres offer group training (asthma schools). In case of an emergency, the child should be admitted to a paediatric department in a hospital. New biomarkers in blood, such as chitinases and periostin, as well as new means of diagnosing allergies, including component resolved diagnosis, which can identify the allergens involved, and basophil allergen threshold sensitivity, offer scope for more precise diagnosis of asthma and its triggers, and predict its severity. Novel treatment possibilities may include macrolide antibiotics and individualized cytokine antagonist therapies (Reference HedlinHedlin, 2014). In-home monitoring using telemedicine also offers future potential (Reference StarmerStarmer et al., 2010).
A child in a critical condition
When a child has an overtly critical condition, such as foreign body aspiration or ingestion, head injury, poisoning, or serious respiratory or cardiac failure, then immediate provision of ambulance services, contactable by phone, must be guaranteed. Ideally, paramedics in the ambulance will be able to initiate immediate life-saving treatment, if that has not already been provided by first-aid. The ambulance should take the child to the emergency unit at the nearest hospital. Ideally, this will have separate provision for emergency care of children.
Countries should have poison control centres offering haemodialysis/adsorption, plasma exchange, and blood exchange at regional or national level, open for consultation by phone 24/7 for medical staff and the public.
Continuous medical education and professional development, including practical training of parents and care givers in emergency situations at both community and hospital level, are essential. It is also important that the equipment provided for emergency responses should cover the entire age spectrum of children. All those staff involved in the emergency response should also be trained in the specific health problems of children. This includes paramedical staff in ambulances but also those who staff emergency telephone services. Teleconsultations can facilitate the emergency care delivered to children in remote areas (Reference Burke and HallBurke & Hall, 2015).
A child with a chronic illness requiring new technologies – type I diabetes mellitus (DM)
Immediate admission of children with diabetic ketoacidosis to hospital is warranted. Insulin, fluids and electrolytes are given while closely monitoring the patient. After stabilization is reached, a well planned preparation of the families for home treatment should be initiated in the paediatric department. Children with type 1 diabetes mellitus are increasingly utilizing continuous subcutaneous insulin infusions (insulin pumps) and continuous glucose monitoring systems (CGMS), both of which have been shown to improve glycaemic control and quality of life if the families are well trained. Proper education for families and providers should be provided to promote successful use of high-tech equipment and will reduce the number of adverse events (Reference ErnstErnst et al., 2016). These children should be closely followed in diabetes outpatient clinics of hospitals or in community centres providing appropriate staff and expertise. Future technology will focus on the new generation of pumps and monitoring (Reference CarchidiCarchidi et al., 2011). Telemedicine applications can facilitate monitoring and adherence to therapy (Reference Burke and HallBurke & Hall, 2015).
Future trends
The care of children has changed remarkably in the past few decades and will continue to do so. Many of the same factors that drove changes in the past will remain important, including advances in technology, models of care, and professional roles. However, it is likely that there will be particularly important advances in information technology, enabling care to be coordinated across many different settings. A number of these key drivers for change are set out in Table 2.3. Beyond these individual drivers, it is clear that there will be a need for much greater integration of services, with child-oriented care delivered jointly by child health professionals working in different locations.
Table 2.3 Future trends influencing the delivery of integrated care to children on five different levels
| Trend | Impact on the future hospital | Example | Challenges |
|---|---|---|---|
| Ongoing medical advances |
|
| Funding of new devices and drugs |
| Health information technology | Paper free children’s hospital | e.g. One record for all health care providers caring for a child with type 1 diabetes mellitus: the primary care taker, hospital paediatric department, subspecialists and community services | Interoperability of electronic health recording systems in hospital and community |
| Innovative models of care (Reference StarmerStarmer et al., 2010) |
| e.g. A child with a neuro-developmental syndrome and epilepsy | Coordination of care across service settings |
| Telemedicine |
| e.g. Monitoring a child with inflammatory bowel disease in a rural area (Reference Burke and HallBurke & Hall, 2015) | Infrastructure |
| Skill mix | Task shifting | e.g. Nurse practitioners in intensive care units (Reference KotzerKotzer, 2005) | New professional hierarchies, team working, quality assurance |
Conclusions and key messages
Existing systems providing secondary health care for children are facing several major challenges. First, there is enormous variation in the quality and nature of care provided for children across the European region, including differences in financial resources, organization of health care, and access to skilled health professionals and advanced technology. Second, the care of sick children has undergone a process of fragmentation, largely reflecting new opportunities to intervene, driven by scientific and technological advances. However, this fragmentation risks being exacerbated by organizational changes in some health systems. Third, although the sick child is on a journey that moves between different levels of care, they and their parents will often be challenged by structural and organizational barriers between primary, secondary, and tertiary care. Finally, all health systems are facing upward pressure on costs, with some aspects of paediatric care, including neonatal intensive care and highly specialized tertiary care, being especially vulnerable.
Many of these challenges apply equally to the provision of hospital care for adults. However, there are some specificities (Box 2.4). All hospitalized children should be admitted to children’s wards and not to adult wards, and those caring for children in hospital face some additional challenges. For example, while it is the child who is being treated in hospital, it is important to find ways to include other members of the family in the process, for example by the creation of family-friendly facilities. It is also the case that children are not simply small adults and many can find the clinical environment frightening, so it is important to incorporate elements of design that create a child-friendly environment (Boxes 2.1 and 2.2). As with any patient with a complex chronic disease, care is increasingly being delivered by multidisciplinary teams but, in the case of children, these teams extend beyond the health sector to the education sector. There are many opportunities for learning from the different models of care seen in Europe but this will require considerable effort to overcome the scarcity of comparative information and of health services research focusing on children’s services.
Box 2.4 Ten rules for the care of children in hospitals
1. The interests of the child should come first, with policies based on an understanding of the importance, and the life course model, of development.
2. Children should be cared for by a team of competent care givers who have been trained in communicating with children and in treating children of all ages.
3. Sick children should be treated in special age-appropriate units and not in adult units.
4. Priority should be given to non-invasive and ambulatory care for children as far as possible.
5. Care givers must have adequate time to communicate with children to strengthen their engagement in the clinical process. Hospital care for children includes support for patients, families and care givers, including the provision of relevant training, a process facilitated by enabling parents to stay with their children.
6. Hospital care for children must be adequately financed and staffed.
7. Child care at all levels should be integrated, taking account of lessons from whole systems thinking.
8. Competition between different care givers and between different institutions is unhelpful and can create unnecessary barriers to the seamless provision of care for children with complex needs.
9. Those providing hospital care for children should participate in research that advances knowledge on the care of children, including both scientific and organizational interventions, and should develop mechanisms to ensure that these advances are incorporated into practice to continuously improve quality of care.
10. Hospital care means a combination of inpatient and outpatient care to avoid fragmented care.
We conclude with a series of challenges for those responsible for the organization and delivery of health care for Europe’s children.
1. How can health systems prepare for the “unknown unknowns” in meeting the health needs of children? There are still many questions about how to translate the explosion in knowledge of the molecular basis of disease, including genomics, proteomics, and metabolomics, into practical solutions that can be applied widely and at scale, and in ways that are affordable.
2. Do stand-alone children’s hospitals have any future? Ageing societies and reduced demands for inpatient care of children, coupled with payment systems that often fail to cover the costs of paediatric care, suggest that most will struggle to survive, with the possible exception of some highly specialized facilities, such as those linked to academic health centres. The challenge facing those stand-alone children’s hospitals that are privately owned are especially great.
3. The motto “sick child in and healthy child out” simplifies the current problems of child health care. Prior to and following a stay in hospital there must be well developed pathways for long-term care, involving a wide range of child health care providers in a variety of settings.
4. How can models of care based on networks and integration across settings be delivered where there is choice of provider? Experience in Germany, for example, suggests that hospitals in such a setting have no incentive to develop initiatives designed to respond to the needs of chronically ill children in and outside hospitals (Reference BusseBusse, 2004).






